Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(2H_2+O_2\rightarrow\left(t^o\right)2H_2O\)
Giả sử có 1 mol O2 => nH2 = 2mol
\(2KMnO_4\rightarrow\left(t^o\right)K_2MnO_4+MnO_2+O_2\)
2 1 ( mol )
\(m_{KMnO_4}=158.2=316g\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
2 2 ( mol )
\(m_{Zn}=65.2=130g\)
\(\dfrac{m_{KMnO_4}}{m_{Zn}}=\dfrac{316}{130}=\dfrac{158}{65}\)

Giả sử có 1 mol \(H_2\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(\dfrac{2}{3}\) 1 1 ( mol )
\(m_{Al}=\dfrac{2}{3}.27=18g\)
\(m_{H_2SO_4}=1.98=98g\)
\(a:b=\dfrac{18}{98}=\dfrac{9}{49}\)

$2Al + 6HCl \to 2AlCl_3 + 3H_2$
$Zn + 2HCl \to ZnCl_2 + H_2$
$n_{Al} = \dfrac{a}{27} (mol) \Rightarrow n_{H_2} = \dfrac{3}{2}n_{Al} = \dfrac{a}{18}(mol)$
$n_{Zn} = \dfrac{b}{65}(mol) \Rightarrow n_{H_2} = n_{Zn} = \dfrac{b}{65}(mol)$
$\Rightarrow \dfrac{a}{18} = \dfrac{b}{65}$
$\Rightarrow \dfrac{a}{b} = \dfrac{18}{65}$

a) Coi X là kim loại R hóa trị n
\(2R + 2nHCl \to 2RCl_n + nH_2\\ n_{H_2} = \dfrac{3,36}{22,4} = 0,15(mol)\\ \Rightarrow n_R = \dfrac{2}{n}n_{H_2} = \dfrac{0,3}{n}(mol)\\ 2R + 2nH_2O \to 2R(OH)_n + nH_2\\ n_{H_2O} = \dfrac{10,8}{18} = 0,6(mol)\\ \Rightarrow n_R = \dfrac{1}{n}n_{H_2O} = \dfrac{0,6}{n}(mol)\\ \)
Suy ra: \(\dfrac{m_1}{m_2} = \dfrac{0,3}{n} : \dfrac{0,6}{n} = \dfrac{1}{2}\)
b)
\(m_2 =2m_1 \\ \Rightarrow C_{M_{HCl\ TN_2}} = 2C_{M_{HCl\ TN_1}} = 0,5.2 = 1M\)

TN1: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
Ta có: \(n_{Fe}=\dfrac{m_1}{56}\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Fe}=\dfrac{m_1}{56}\left(mol\right)\)
TN2: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
Ta có: \(n_{Al}=\dfrac{m_2}{27}\left(mol\right)\)
Theo PT: \(n_{H_2}=\dfrac{3}{2}n_{Al}=\dfrac{m_2}{18}\left(mol\right)\)
Mà: \(V_2=1,5V_1\Rightarrow\dfrac{V_1}{V_2}=\dfrac{1}{1,5}=\dfrac{2}{3}\)
\(\Rightarrow\dfrac{n_1}{n_2}=\dfrac{n_{H_2\left(Fe\right)}}{n_{H_2\left(Al\right)}}=\dfrac{2}{3}\) \(\Rightarrow\dfrac{\dfrac{m_1}{56}}{\dfrac{m_2}{18}}=\dfrac{2}{3}\)
\(\Rightarrow\dfrac{m_1}{m_2}=\dfrac{56}{27}\)

Gọi \(n_{H_2}=x\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(\dfrac{2}{3}x\) x ( mol )
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(x\) x ( mol )
\(a:b=\dfrac{\dfrac{2}{3}x.27}{65x}=\dfrac{18x}{65x}=\dfrac{18}{65}\)

2Al+ 3H2SO4-> Al2(SO4)3 +3H2
2a 3a Zn+H2SO4 -> ZnSO4 +H2 b b Theo bài ra ta có b=3a => a:b= 1:3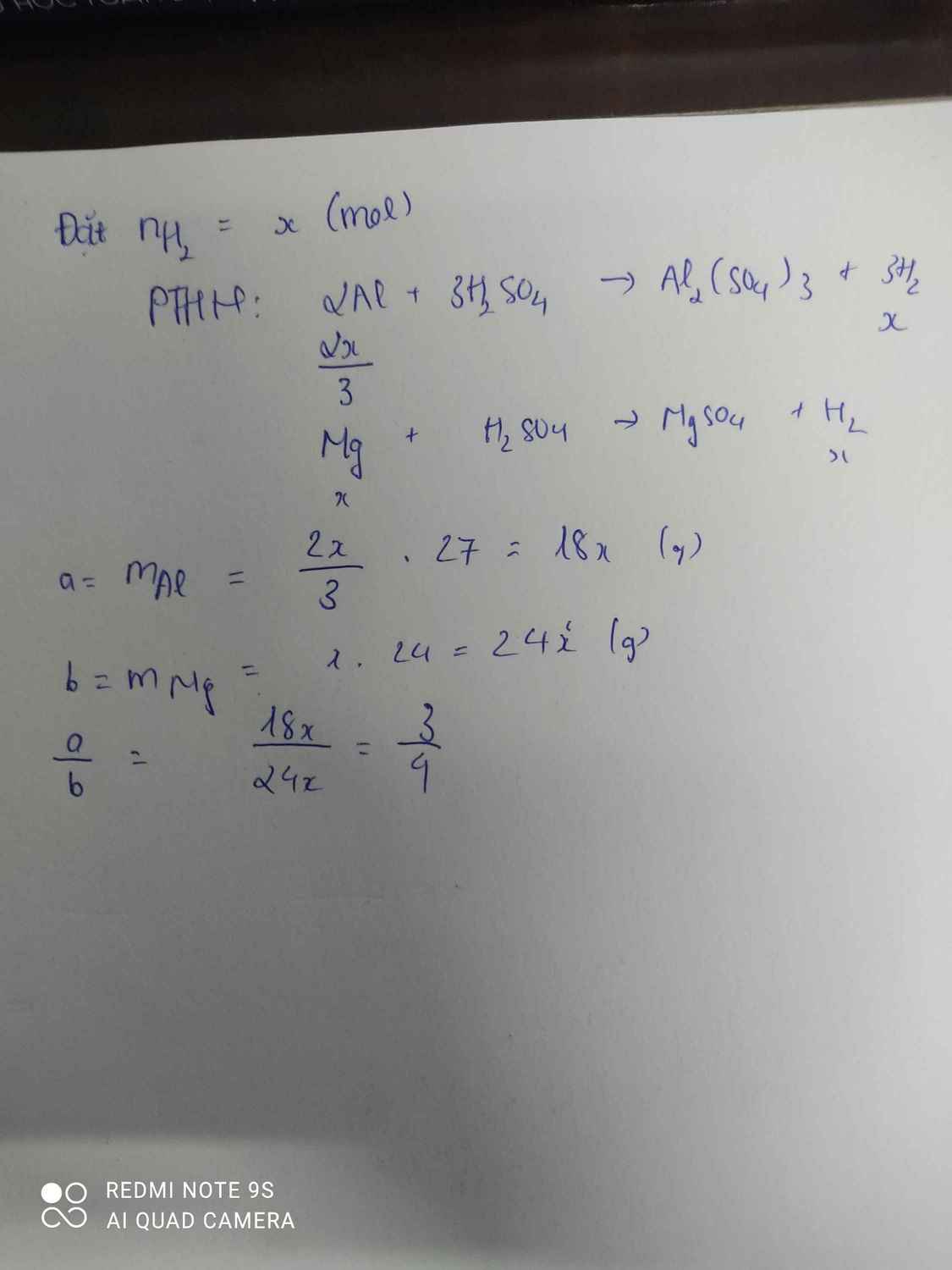
giả sử đều sinh ra 1(mol) h2
pthh:
2Al+6HCl..==>2AlCl3.+3H2
2/3....................................1
Zn+2HCl...==>..ZnCl2.+.H2
1......................................1
m1:m2=18:65