Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

nO2 = 3,36 : 22,4 = 0,15 (mol)
pthh : 2Mg + O2 -t--> 2MgO
0,3<----0,15---> 0,3 (mol)
=> mMg= 0,3 . 24 = 7,2 (g)
=> mMgO = 0,3 . 40 =12 (g)
pthh : 2KMnO4 -t--> K2MnO4 + MnO2 + O2
0,3<-------------------------------------0,15 (mol)
=> mKMnO4 = 0,3 . 158 = 47,4 (g)

a.\(n_{Zn}=\dfrac{m_{Zn}}{M_{Zn}}=\dfrac{19,5}{65}=0,3mol\)
\(2Zn+O_2\rightarrow\left(t^o\right)2ZnO\)
0,3 0,15 ( mol )
\(V_{O_2}=n_{O_2}.22,4=0,15.22,4=3,36l\)
b.\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
0,1 0,15 ( mol )
\(m_{KClO_3}=n_{KClO_3}.M_{KClO_3}=0,1.122,5=12,25g\)
a) PTHH: 2Zn + O2 → 2ZnO
2 1 2
0,3 0,15 0,3
nZn = \(\dfrac{m}{M}\) = \(\dfrac{19,5}{65}\) = 0,3 (mol)
mO2 = n.M = 0,15 . 16 = 2,4 (g)
VO2 = m . 22,4 = 2,4 . 22,4 = 53,76 (l)
b) 2KClO3 → 2KCl + 3O2 ↑
0,1 0,1 0,15
mKClO3 = n . M = 0,1 . 122,5 = 12,25 (g)

a)\(n_{Al}=\dfrac{5,4}{27}=0,2\left(m\right)\)
\(PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
tỉ lệ :4 3 2
số mol :0,2 0,15 0,1
\(V_{O_2}=0,15.22,4=3,36\left(l\right)\)
b)\(m_{Al_2O_3}=0,1.102=10,2\left(g\right)\)
c)\(PTHH:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
tỉ lệ :2 1 1 1
số mol :0,3 0,15 0,15 0,15
\(m_{KMnO_4}=0,3.126=37,8\left(g\right)\)

Bài 1:
\(a,2Cu+O_2\underrightarrow{t^o}2CuO\)
b, \(n_{O_2}=\dfrac{1,12}{32}=0,035mol\)
\(n_{Cu}=\dfrac{6,4}{64}=0,1mol\)
\(\dfrac{0,1}{2}>\dfrac{0,035}{1}\) => Cu dư, O2 đủ
\(n_{Cu}\left(dư\right)=0,1-0,07=0,039\left(mol\right)\)
c, \(m_{CuO}=0,07.80=5,6g\)
Bài 2:
\(n_{Al}=\dfrac{13,5}{27}=0,5mol\)
\(n_{O_2}=\dfrac{6,67}{32}=0,21\left(mol\right)\)
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
\(\dfrac{0,5}{4}>\dfrac{0,21}{3}\) => Al dư, O2 đủ
\(n_{Al_2O_3}=\dfrac{2}{3}.0,21=0,14\left(mol\right)\)
\(m_{Al_2O_3}=0,14.102=14,28g\)

a, PT: \(2Zn+O_2\underrightarrow{t^o}2ZnO\) - pư hóa hợp.
b, \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{Zn}=0,1\left(mol\right)\Rightarrow V_{O_2}=0,1.22,4=2,24\left(l\right)\)
c, \(n_{H_2SO_4}=\dfrac{9,8}{98}=0,1\left(mol\right)\)
PT: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
Xét tỉ lệ: \(\dfrac{0,2}{1}>\dfrac{0,1}{1}\), ta được Zn dư.
Theo PT: \(n_{H_2}=n_{H_2SO_4}=0,1\left(mol\right)\Rightarrow V_{H_2}=0,1.22,4=2,24\left(l\right)\)
\(n_{ZnSO_4}=n_{H_2SO_4}=0,1\left(mol\right)\Rightarrow m_{ZnSO_4}=0,1.161=16,1\left(g\right)\)

a) PTHH: \(Zn+\dfrac{1}{2}O_2\xrightarrow[]{t^o}ZnO\)
b) Ta có: \(n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\) \(\Rightarrow n_{O_2}=0,15\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,15\cdot22,4=3,36\left(l\right)\)
c) PTHH: \(KClO_3\xrightarrow[MnO_2]{t^o}KCl+\dfrac{3}{2}O_2\uparrow\)
Theo PTHH: \(n_{KClO_3}=0,1\left(mol\right)\) \(\Rightarrow m_{KClO_3}=0,1\cdot122,5=12,25\left(g\right)\)

\(n_{H_2}=\dfrac{2,9748}{24,79}=0,12(mol)\\ 2AL+6HCl\to 2AlCl_3+3H_2\\ \Rightarrow n_{Al}=n_{AlCl_3}=0,08(mol);n_{HCl}=0,24(mol)\\ a,m_{Al}=0,08.2=2,16(g)\\ m_{HCl}=0,24.36,5=8,76(g)\\ b,m_{AlCl_3}=0,08.133,5=10,68(g)\\ c,2H_2+O_2\xrightarrow{t^o}2H_2O\\ \Rightarrow n_{H_2O}=0,12(mol)\\ \Rightarrow m_{H_2O}=0,12.18=2,16(g)\)
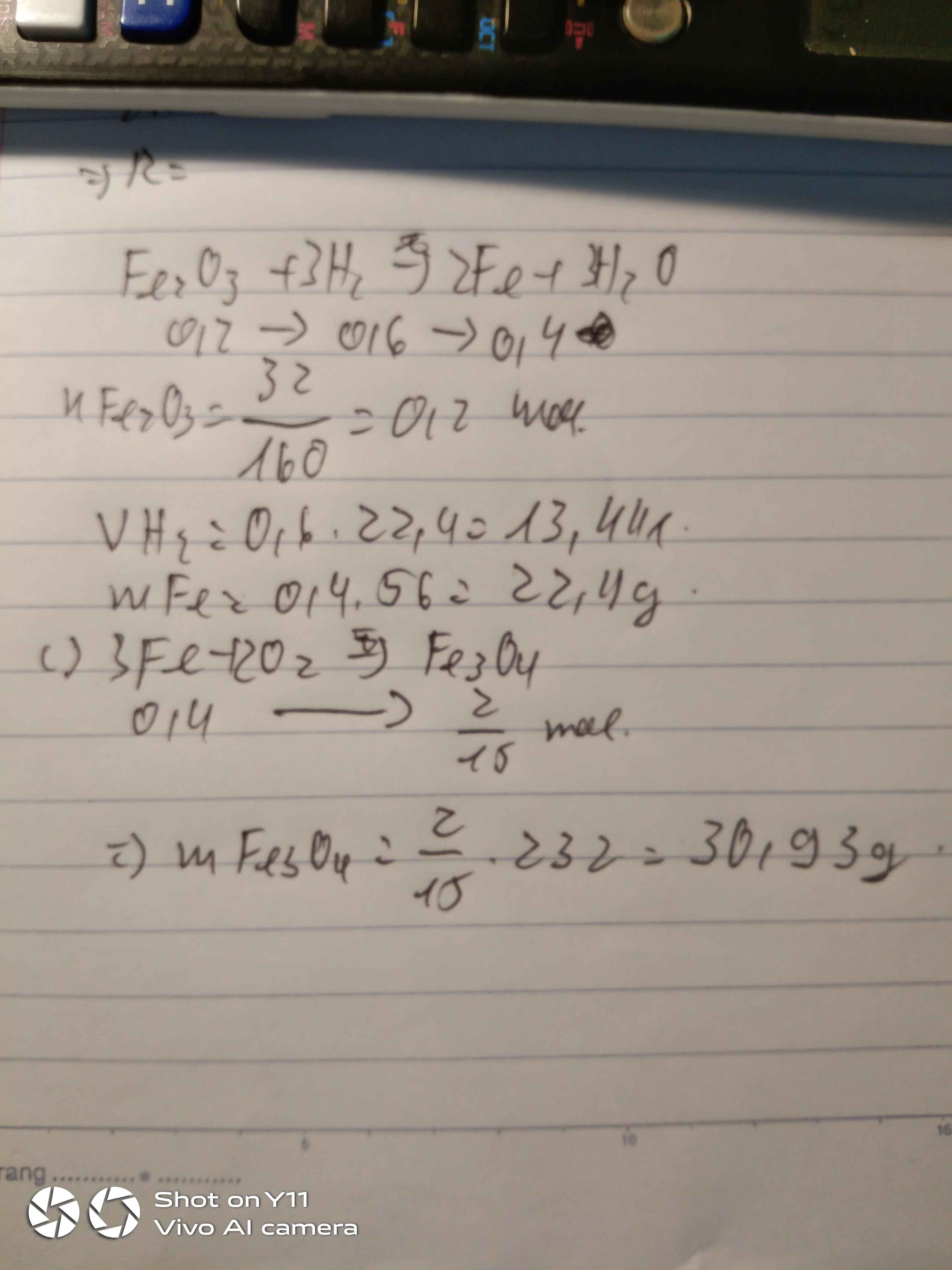

a. \(n_{O_2}=\dfrac{3.36}{22,4}=0,15\left(mol\right)\)
PTHH : 2Zn + O2 -------to------> 2ZnO
0,3 0,15 0,15
\(m_{Zn}=65.0,3=19,5\left(g\right)\)
b. \(m_{ZnO}=0,15.81=12,15\left(g\right)\)
c. PTHH : 2KMnO4 ---to---> K2MnO4 + MnO2 + O2
0,3 0,15
\(m_{KMnO_4}=158.0,3=47,4\left(g\right)\)
\(n_{O_2}=\dfrac{33,6}{22,4}=1,5\left(mol\right)\)
PTHH: 2Zn + O2 --to--> 2ZnO
3 1,5 3
\(\rightarrow\left\{{}\begin{matrix}m_{Zn}=3.65=195\left(g\right)\\m_{ZnO}=81.3=243\left(g\right)\end{matrix}\right.\)
2KMnO4 --to--> K2MnO4 + MnO2 + O2
6 3
=> mKMnO4 = 6.158 = 948 (g)