Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\\ a,2Mg+O_2\rightarrow\left(t^o\right)2MgO\\ n_{O_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ V_{O_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ b,2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\\ n_{KClO_3}=\dfrac{0,05.2}{3}=\dfrac{1}{30}\left(mol\right)\\ \Rightarrow m_{KClO_3}=\dfrac{122,5}{30}=\dfrac{49}{12}\left(g\right)\)

mMg = 3.6/24 = 0.15 (mol)
2Mg + O2 -to-> 2MgO
0.15__0.075____0.15
mMgO= 0.15*40 = 6 (g)
VO2 = 0.075*22.4 = 1.68 (l)
2KClO3 -to-> 2KCl + 3O2
0.05_______________0.075
mKClO3 = 0.05*122.5 = 6.125 (g)
PTHH: \(2Mg+O_2\underrightarrow{t^o}2MgO\)
a+b) Ta có: \(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{O_2}=0,075\left(mol\right)\\n_{MgO}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{O_2}=0,075\cdot22,4=1,68\left(l\right)\\m_{MgO}=0,15\cdot40=6\left(g\right)\end{matrix}\right.\)
c) PTHH: \(2KClO_3\xrightarrow[MnO_2]{t^o}2KCl+3O_2\uparrow\)
Theo PTHH: \(n_{KClO_3}=0,05\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=0,05\cdot122,5=6,125\left(g\right)\)

PTHH : 2Cu + O2 ---> 2CuO (1)
2KMnO4 ---> K2MnO4 + MnO2 + O2 (2)
Từ gt => nCu =16:64 = 0,25 (mol)
Từ (1) và gt => nCu = nCuO = 2 nO2
=> nCuO = 0,25 mol
nO2 = 0,125 mol
=> mCuO = 0,25 x 80 = 20 (g)
VO2 = 0,125 x 22,4 = 2,8 (l)
Từ (2) => nKMnO4 = 2 nO2
=> nKMnO4 = 0,25
=> mKMnO4 = 0,25 x 158 = 39,5(g)

1. a. \(PTHH:2Mg+O_2\overset{t^o}{--->}2MgO\left(1\right)\)
b. Ta có: \(n_{Mg}=\dfrac{24}{24}=1\left(mol\right)\)
Theo PT(1): \(n_{O_2}=\dfrac{1}{2}.n_{Mg}=\dfrac{1}{2}.0,1=0,5\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,5.22,4=11,2\left(lít\right)\)
c. \(PTHH:2KClO_3\xrightarrow[t^o]{MnO_2}2KCl+3O_2\left(2\right)\)
Theo PT(2): \(n_{KClO_3}=\dfrac{2}{3}.n_{O_2}=\dfrac{2}{3}.0,5=\dfrac{1}{3}\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=\dfrac{1}{3}.122,5=40,83\left(g\right)\)
2. \(PTHH:3Fe+2O_2\overset{t^o}{--->}Fe_3O_4\)
Ta có: \(n_{Fe_3O_4}=\dfrac{2,32}{232}=0,01\left(mol\right)\)
a. Theo PT: \(n_{Fe}=3.n_{Fe_3O_4}=0,01.3=0,03\left(mol\right)\)
\(\Rightarrow m_{Fe}=0,03.56=1,68\left(g\right)\)
b. Theo PT: \(n_{O_2}=2.n_{Fe_3O_4}=2.0,01=0,02\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,02.22,4=0,448\left(lít\right)\)

a, \(2Cu+O_2\underrightarrow{t^o}2CuO\)
b, \(n_{Cu}=\dfrac{16}{64}=0,25\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{Cu}=0,125\left(mol\right)\Rightarrow V_{O_2}=0,125.22,4=2,8\left(l\right)\)
c, \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Theo PT: \(n_{KMnO_4}=2n_{O_2}=0,25\left(mol\right)\Rightarrow m_{KMnO_4}=0,25.158=39,5\left(g\right)\)

a) \(n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH: 3Fe + 2O2 --to--> Fe3O4
0,3<---0,2-------->0,1
=> m = 0,3.56 = 16,8 (g)
b) mFe3O4 = 0,1.232 = 23,2 (g)
c) Vkk = 4,48 : 20% = 22,4 (l)
nO2 = 4,48/22,4 = 0,2 (mol)
PTHH: 3Fe + 2O2 -> (t°) Fe3O4
Mol: 0,3 <--- 0,2 ---> 0,1
mFe = 0,3 . 56 = 16,8 (g)
mFe3O4 = 0,1 . 232 = 23,2 (g)
Vkk = 4,48 . 5 = 22,4 (l)

a) \(n_{O_2}=\dfrac{11,2.20\%}{22,4}=0,1\left(mol\right)\)
PTHH: 2Mg + O2 --to--> 2MgO
0,2<--0,1--------->0,2
=> mMg = 0,2.24 = 4,8 (g)
b) nMgO = 0,2.40 = 8 (g)
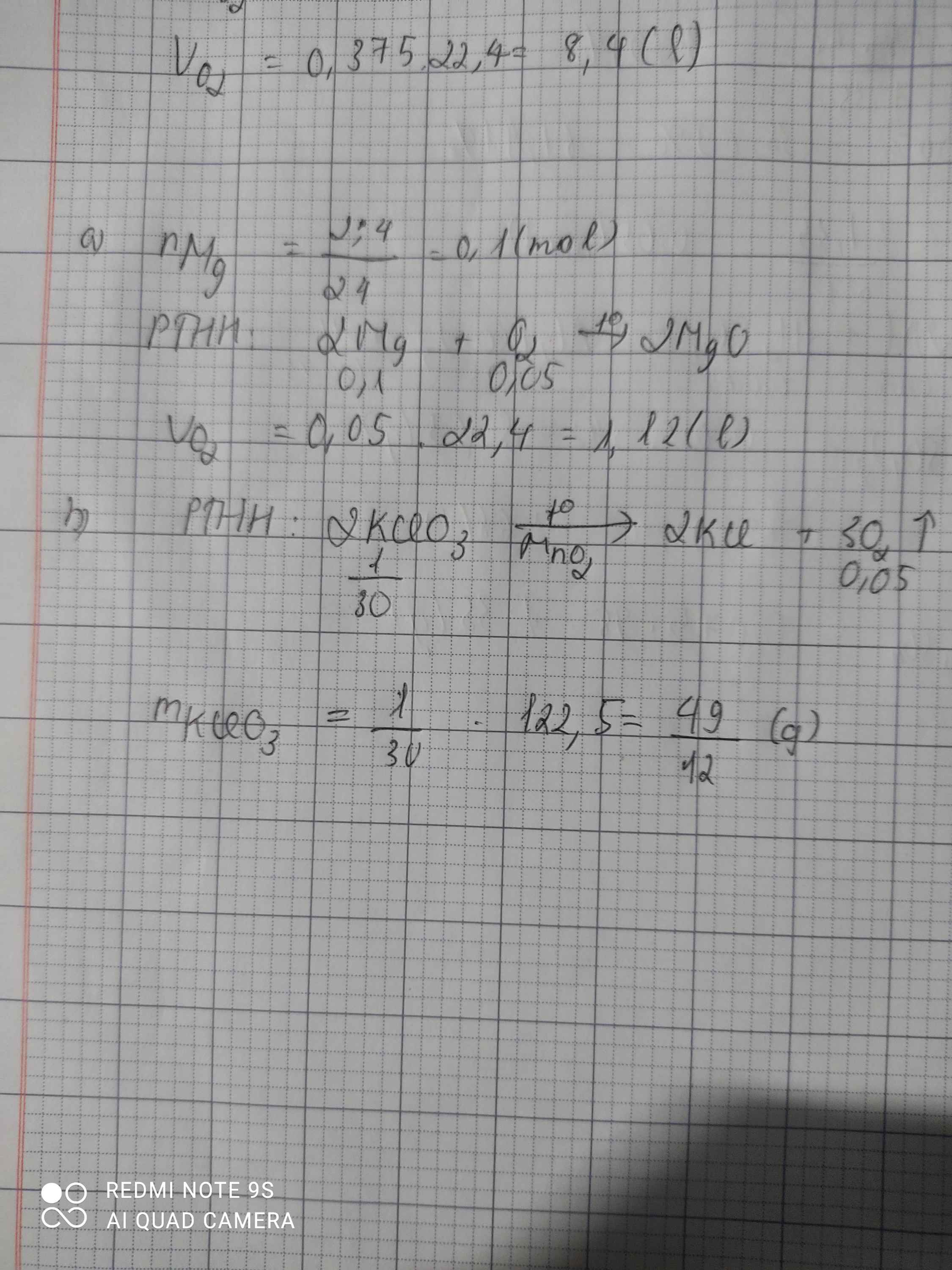
a)
\(n_{Mg}=\dfrac{12}{24}=0,5\left(mol\right)\)
PTHH: 2Mg + O2 --to--> 2MgO
______0,5-->0,25---->0,5
=> VO2 = 0,25.22,4 = 5,6 (l)
=> mMgO = 0,5.40 = 20 (g)
b)
\(n_{O_2}=0,25=>n_{CO_2}=0,25\)
=> mCO2 = 0,25.44 = 11 (g)