Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, Gọi \(m_{NaCl\left(thêm\right)}=a\left(g\right)\)
\(m_{NaCl\left(bđ\right)}=5\%.100=5\left(g\right)\\ \Rightarrow C\%_{NaCl}=\dfrac{5+a}{100+a}.100\%=5,5\%\\ \Leftrightarrow a=0,53\left(g\right)\)
b, \(m_{NaCl}=58,5.5,5\%=3,2175\left(g\right)\\ n_{NaCl}=\dfrac{3,2175}{58,5}=0,055\left(mol\right)\)
PTHH: NaCl + AgNO3 ---> AgCl↓ + NaNO3
0,055-->0,055------>0,055---->0,055
\(m_{AgCl}=0,055.143,5=7,8925\left(g\right)\\ m_{ddY}=58,5+200-7,8925=250,6075\left(g\right)\\ \Rightarrow C\%_{NaNO_3}=\dfrac{0,055.85}{250,6075}.100\%=1,87\%\)

\(n_{Al_2O_3}=\dfrac{5.1}{102}=0.05\left(mol\right)\)
\(Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(0.05...........0.15...............0.05\)
\(C_{M_{H_2SO_4}}=\dfrac{0.15}{0.2}=0.75\left(M\right)\)
\(C_{M_{Al_2\left(SO_4\right)_3}}=\dfrac{0.05}{0.2}=0.25\left(M\right)\)

\(n_{CuSO_4}=\dfrac{160.10\%}{160}=0,1\left(mol\right)\)
\(n_{NaOH}=\dfrac{150.8\%}{40}=0,3\left(mol\right)\)
PTHH: CuSO4 + 2NaOH --> Cu(OH)2 + Na2SO4
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,3}{2}\) => CuSO4 hết, NaOH dư
PTHH: CuSO4 + 2NaOH --> Cu(OH)2 + Na2SO4
0,1------>0,2------->0,1------->0,1
=> m = 0,1.98 = 9,8 (g)
\(\left\{{}\begin{matrix}m_{NaOH_{dư}}=\left(0,3-0,2\right).40=4\left(g\right)\\m_{Na_2SO_4}=0,1.142=14,2\left(g\right)\end{matrix}\right.\)
mdd sau pư = 160 + 150 - 9,8 = 300,2 (g)
\(\left\{{}\begin{matrix}C\%_{NaOH_{dư}}=\dfrac{4}{300,2}.100\%=1,33\%\\C\%_{Na_2SO_4}=\dfrac{14,2}{300,2}.100\%=4,73\%\end{matrix}\right.\)
\(m_{CuSO_4}=\dfrac{160.10}{100}=16\left(g\right)\\ n_{NaOH}=\dfrac{8.150}{100}=12\left(g\right)\\ \rightarrow\left\{{}\begin{matrix}n_{CuSO_4}=\dfrac{16}{160}=0,1\left(mol\right)\\n_{NaOH}=\dfrac{12}{40}=0,3\left(mol\right)\end{matrix}\right.\)
PTHH: 2NaOH + CuSO4 ---> Cu(OH)2 + Na2SO4
LTL: \(0,1< \dfrac{0,3}{2}\rightarrow\) NaOH dư
Theo pt: \(\left\{{}\begin{matrix}n_{NaOH\left(pư\right)}=\dfrac{1}{2}n_{CuSO_4}=2.0,1=0,2\left(mol\right)\\n_{Na_2SO_4}=n_{Cu\left(OH\right)_2}=n_{CuSO_4}=0,1\left(mol\right)\end{matrix}\right.\)
\(\rightarrow m=0,1.98=9,8\left(g\right)\\ m_{dd}=160+150-9,8=300,2\left(g\right)\\ \rightarrow\left\{{}\begin{matrix}C\%_{NaOH\left(dư\right)}=\dfrac{\left(0,3-0,2\right).40}{300,2}=1,33\%\\C\%_{Na_2SO_4}=\dfrac{0,1.142}{300,2}=4,73\%\end{matrix}\right.\)

a/
\(n_{Na_2O}=\dfrac{9,3}{62}=0,15\left(mol\right)\)
\(Na_2O+H_2O\rightarrow2NaOH\)
0,15 0,3 (mol)
\(m_{NaOH}=0,3.40=12\left(g\right)\)
\(m_A=90,7+9,3=100\left(g\right)\)
\(C\%_{NaOH}=\dfrac{12}{100}.100\%=12\%\)
b/
m\(_{FeSO_4}=\dfrac{16.200}{100}=32\left(g\right)\)
\(\rightarrow m_{FeSO_4}=\dfrac{32}{152}=\dfrac{4}{19}\left(mol\right)\)
\(2NaOH+FeSO_4\rightarrow Na_2SO_4+Fe\left(OH\right)_2\downarrow\)
bđ: 0,3 \(\dfrac{4}{19}\) 0 0 (mol)
pư: 0,3 0,15 0,15 0,15 (mol)
dư: 0 \(\dfrac{23}{380}\) (mol)
\(m_{Fe\left(OH\right)_2}=0,15.90=13,5\left(g\right)\)
\(m_C=100+200-13,5=286,5\left(g\right)\)
\(m_{Na_2SO_4}=0,15.142=21,3\left(g\right)\)
\(\rightarrow C\%_{Na_2SO_4}=\dfrac{21,3}{286,5}.100\%\approx7,4\%\)
\(m_{FeSO_4\left(dư\right)}=\dfrac{23}{380}.152=9,2\left(g\right)\)
\(\rightarrow C\%_{FeSO_4\left(dư\right)}=\dfrac{9,2}{286,5}.100\%\approx3,2\%\)

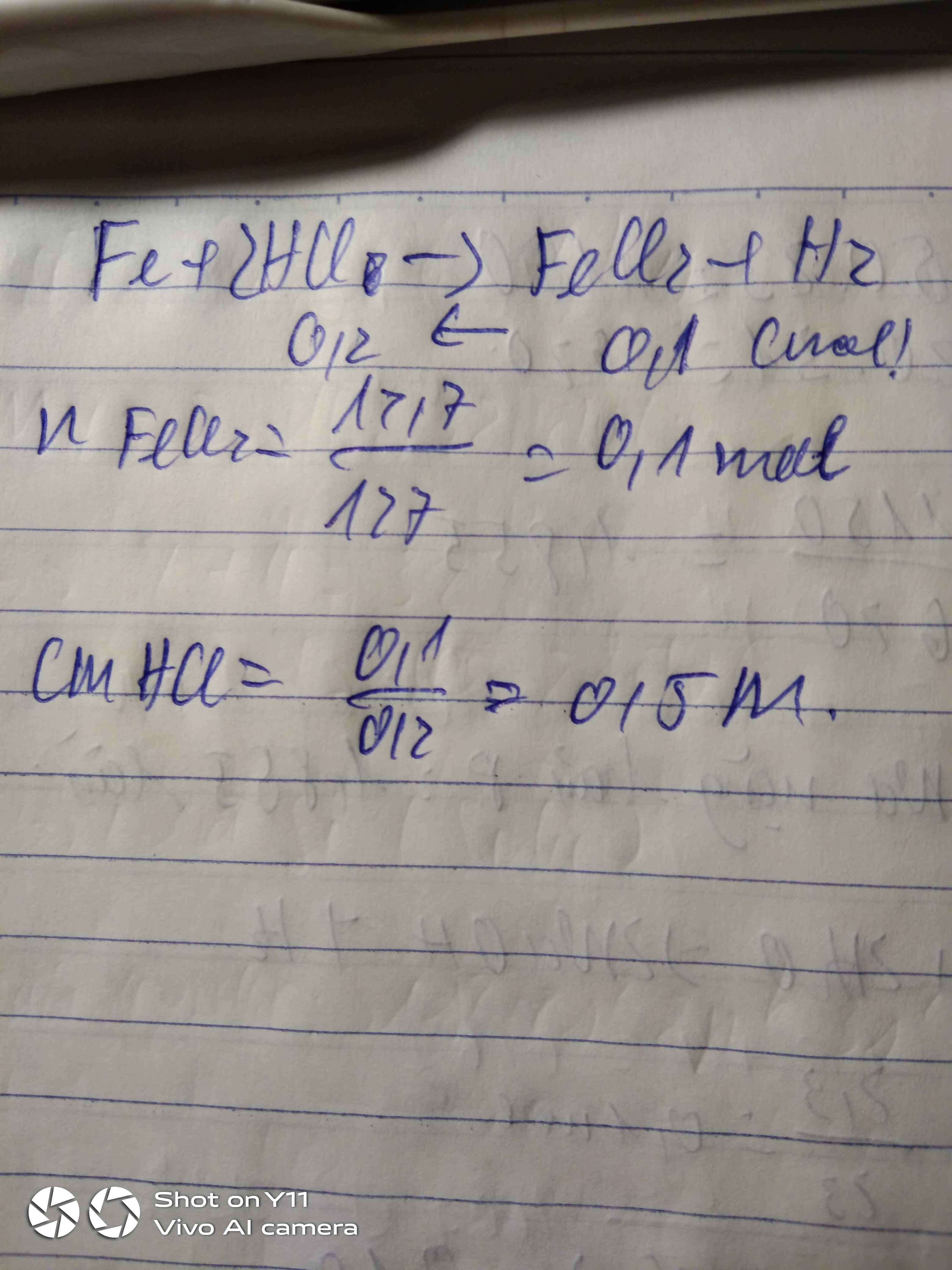
Giả sử có 1 mol M2CO3.10H2O \(\Rightarrow\) nM2CO3 = 1mol
M2CO3 + BaCl2 \(\rightarrow\) BaCO3 + 2MCl (1)
1mol 1mol 1 mol 2mol
\(\Rightarrow\) m ddBaCl2 = 1.208. \(\frac{100}{5}\)= 4160 (g)
\(\Rightarrow\) \(\frac{2.\left(M+35,5\right)}{4160+1.\left(2M+240\right)-1.197}\) = \(\frac{2,7536}{100}\)
\(\Rightarrow\) M = 23 \(\Rightarrow\) M là Na
\(\Rightarrow\) M2CO3.10H2O