Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

1) \(\left(C_{17}H_{35}COO\right)_3C_3H_5+3NaOH\rightarrow3C_{17}H_{35}COONa+C_3H_5\left(OH\right)_3\)
2) \(\left(RCOO\right)_3C_3H_5+3NaOH\rightarrow3RCOONa+C_3H_5\left(OH\right)_3\)
3) \(2CH_3COOH+Na_2CO_3\rightarrow2CH_3COONa+CO_2+H_2O\)
4) \(CH_3COOH+NaOH\rightarrow CH_3COONa+H_2O\)
5) \(2CH_3COONa+H_2SO_4\rightarrow Na_2SO_4+2CH_3COOH\)

\(a) 2Na + 2C_2H_5OH \to 2C_2H_5ONa + H_2\\ b) CH_3COOH + NaOH \to CH_3COONa + H_2O\\ c) (RCOO)_3C_3H_5 +3H_2O \to 3RCOOH + C_3H_5(OH)_3\\ d) C_2H_5OH + CH_3COOH \to CH_3COOC_2H_5 + H_2O\\ e) C_2H_2 + 2Br_2 \to C_2H_2Br_4\\ d) CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ g) (RCOO)_3C_3H_5 + 3NaOH \to 3RCOONa + C_3H_5(OH)_3\)

– Tác dụng với Na (chỉ có rượu hoặc axit)
C2H5OH + Na → C2H5ONa + ½ H2
CH3COOH + Na → CH3COONa + ½ H2
– Tác dụng với NaOH (chỉ có axit hoặc este)
CH3COOH + NaOH → CH3COONa + H2O
(C17H35COO)3C3H5 + 3NaOH →3C17H35COONa + C3H5(OH)3

a, \(2CH_3COOH+CuO\rightarrow\left(CH_3COO\right)_2Cu+H_2O\)
b, \(C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\)
c, \(C_6H_{12}O_6+2AgNO_3+3NH_3\underrightarrow{t^o}C_5H_{11}O_5COONH_4+2Ag+2NH_4NO_3\)
d, \(\left(RCOO\right)_3C_3H_5+3NaOH\underrightarrow{t^o}3RCOONa+C_3H_5\left(OH\right)_3\)
e, \(2CH_3COOH+Na_2CO_3\rightarrow2CH_3COONa+CO_2+H_2O\)
f, \(H_2SO_4+2CH_3COONa\rightarrow2CH_3COOH+Na_2SO_4\)

CH3COOH + KOH → CH3COOK + H2O
CH2=CHCOOCH3 + KOH → CH2=CH–COOK + CH3OH
(C15H31COO)3C3H5 + 3KOH → 3C15H31COOK + C3H5(OH)3
Al4C3 + 12H2O → 4Al(OH)3 + 3CH4
Al(OH)3 + KOH → KAlO2 + 2H2O
C2H5Cl + KOH → KCl + C2H5OH

\(n_{NaOH}=\dfrac{12}{40}=0,3\left(mol\right)\\ PTHH:\left(RCOO\right)_3C_3H_5+3NaOH\rightarrow3RCOONa+C_3H_5\left(OH\right)_3\\ \Rightarrow n_{C_3H_5\left(OH\right)_3}=\dfrac{1}{3}n_{NaOH}=0,1\left(mol\right)\\ \Rightarrow m_{C_3H_5\left(OH\right)_3}=0,1\cdot92=9,2\left(g\right)\\ \Rightarrow m_{RCOONa}=m_{\left(RCOO\right)_3C_3H_5}+m_{NaOH}-m_{C_3H_5\left(OH\right)_3}=89+12-9,2=91,8\left(g\right)\)

\(\left(C_{17}H_{35}COO\right)_3C_3H_5+3NaOH\rightarrow3C_{17}H_{35}COONa+C_3H_5\left(OH\right)_3\\ n_{\left(C_{17}H_{35}COO\right)_3C_3H_5}=\dfrac{8,9}{890}=0,01\left(mol\right)\\ n_{C_{17}H_{35}COONa}=3n_{\left(C_{17}H_{35}COO\right)_3C_3H_5}=0,01.3=0,03\left(mol\right)\\ \Rightarrow m_{C_{17}H_{35}COONa}=0,03.306=9,18\left(kg\right)\\ \Rightarrow m_{xp}=\dfrac{9,18}{60\%}=15,3\left(kg\right)\)
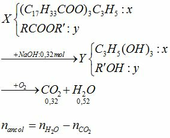
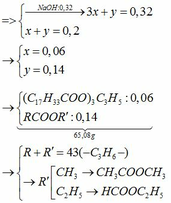
(Ch3COO)3C3H5 + 3NaOH → (Ch3COONa)3 + C3H5(OH)3
(C17H35COO)3C3H5 + 3H2O → C3H5(OH)3 + 3C17H35COOH
(C17H33COO)3C3H5 + 3NAOH → 3C17H33COONA + C3H5(OH)3
CH3COOC2H5 + KOH ---(H2O;t^o------> CH3COOK + C2H5OH