Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{NaCl\left(pư\right)}=\dfrac{12,87.90\%}{58,5}=0,198\left(mol\right)\)
PTHH: 2NaCl + H2SO4(đ) --to--> Na2SO4 + 2HCl
0,198--------------------->0,099------>0,198
=> mNa2SO4 = 0,099.142 = 14,058 (g)
VHCl = 0,198.22,4 = 4,4352 (l)

H2 + Cl2 => 2HCl
Bđ: 3___4
Pư:3*0.9_2.7___5.4
Kt : 0.3__1.3____5.4
V = 0.3 + 1.3 + 5.4 = 7(l)

PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
a) Ta có: \(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)=n_{Zn}\)
\(\Rightarrow\%m_{Zn}=\dfrac{0,4\cdot65}{36,2}\cdot100\%\approx71,23\%\) \(\Rightarrow\%m_{Al_2O_3}=28,77\%\)
c) Ta có: \(n_{Al_2O_3}=\dfrac{36,2-0,4\cdot65}{102}=0,1\left(mol\right)\)
Theo PTHH: \(n_{HCl}=2n_{Zn}+6n_{Al_2O_3}=1,4\left(mol\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{1,4\cdot36,5}{10\%}=511\left(g\right)\) \(\Rightarrow V_{ddHCl}=\dfrac{511}{1,1}\approx464,5\left(ml\right)=0,4645\left(l\right)\)
c) Theo PTHH: \(\left\{{}\begin{matrix}n_{ZnCl_2}=0,4\left(mol\right)\\n_{AlCl_3}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{ZnCl_2}}=\dfrac{0,4}{0,4645}\approx0,86\left(M\right)\\C_{M_{AlCl_3}}=\dfrac{0,2}{0,4645}\approx0,43\left(M\right)\end{matrix}\right.\)

Bạn sửa đề thành 7.84 (l) nha
nH2 = 7.84/22.4 = 0.35 (mol)
Đặt :
nFe = x mol
nMg = y mol
mhh = 56x + 24y = 14.8 g (1)
Fe + H2SO4 => FeSO4 + H2
Mg + H2SO4 => MgSO4 + H2
nH2 = x + y = 0.35 (2)
(1) , (2) :
x = 0.2 => mFe = 0.2*56 = 11.2 g
y = 0.15 => mMg = 0.15*24=3.6 g
%Fe = 75.67%
%Mg = 24.33%
Gọi nFe = a (mol); nMg = b (mol)
=> 56a + 24b = 14,8
\(n_{H_2}=\dfrac{7,89}{22,4}=\dfrac{789}{2240}\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
______a-------------------------->a _________(Mol)
Mg + 2HCl --> MgCl2 + H2
_b--------------------------->b_______(mol)
=> \(\left\{{}\begin{matrix}56a+24b=14,8\\a+b=\dfrac{789}{2240}\end{matrix}\right.\) => \(\left\{{}\begin{matrix}a=\dfrac{1777}{8960}\left(mol\right)\\b=\dfrac{197}{1280}\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{\dfrac{1777}{8960}.56}{14,8}.100\%=75\%\\\%m_{Mg}=\dfrac{\dfrac{197}{1280}.24}{14,8}.100\%=25\%\end{matrix}\right.\)

Đáp án C
nNaCl =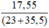 = 0,3 mol
= 0,3 mol
2NaCl + H2SO4 → Na2SO4 + 2HCl
0,3 (mol) → 0,3 (mol)
H% = 90% => nHCl = 0,3. = 0,27 (mol)
= 0,27 (mol)
VHCl = 0,27.22,4 = 6,048 (lít)