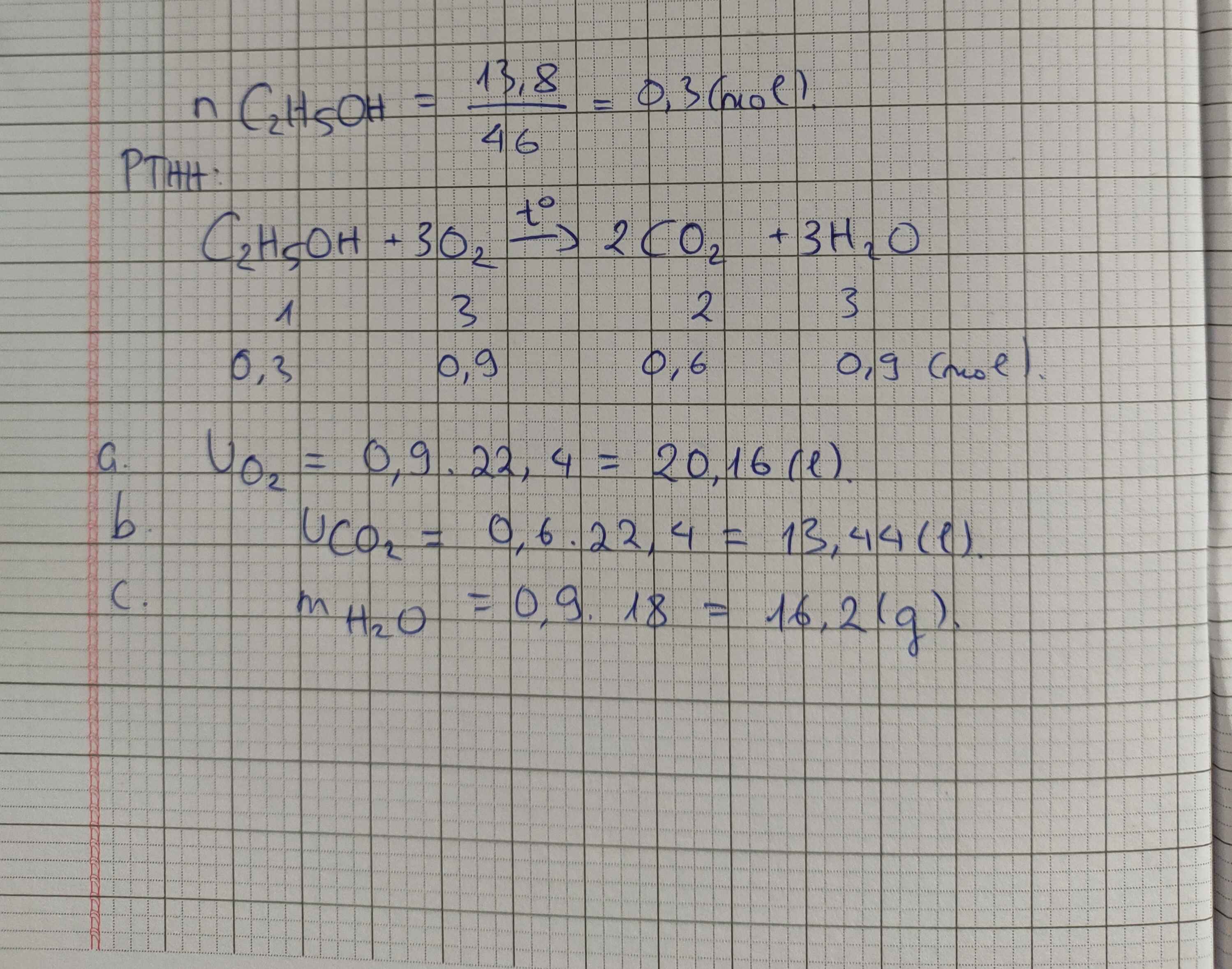Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PT: \(C_2H_6O+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
Theo PT: \(n_{CO_2}=\dfrac{2}{3}n_{O_2}=\dfrac{2}{15}\left(mol\right)\Rightarrow V_{CO_2}=\dfrac{2}{15}.22,4=\dfrac{224}{75}\left(l\right)\)
b, \(n_{C_2H_6O\left(LT\right)}=\dfrac{1}{3}n_{O_2}=\dfrac{1}{15}\left(mol\right)\)
Mà: H = 90%
\(\Rightarrow n_{C_2H_6O\left(TT\right)}=\dfrac{\dfrac{1}{15}}{90\%}=\dfrac{2}{27}\left(mol\right)\)
\(\Rightarrow m_{C_2H_6O}=\dfrac{2}{27}.46=\dfrac{92}{27}\left(g\right)\)

\(n_{C2H5OH}=\dfrac{34,5}{46}=0,75\left(mol\right)\)
Pt : \(C_2H_5OH+3O_2\xrightarrow[]{t^o}2CO_2+3H_2O\)
0,75 1,5
\(CH_3COOH+C_2H_5OH⇌CH_3COOC_2H_5+H_2O\)
0,75 0,75 0,75
a) \(V_{CO2\left(dktc\right)}=1,5.22,4=33,6\left(l\right)\)
b) \(m_{CH3COOC2H5\left(lt\right)}=0,75.88=66\left(g\right)\)
⇒\(m_{CH3COOC2H5\left(tt\right)}=\) \(66.90\%=59,4\left(g\right)\)
Chúc bạn học tốt
À , ý b) trong lúc làm bài bạn bổ sung vào giúp mình nhé

\(n_{C_2H_5OH}=\dfrac{23}{46}=0,5\left(mol\right)\\a, PTHH:C_2H_5OH+3O_2\rightarrow\left(t^o\right)2CO_2+3H_2O\\ b,n_{O_2}=3.0,5=1,5\left(mol\right)\\ V_{O_2\left(đktc\right)}=22,4.1,5=33,6\left(l\right)\\ c,V_{C_2H_5OH}=46\%.100=46\left(ml\right)\\ C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\\ n_{C_2H_5OH}=\dfrac{0,8.46}{46}=0,8\left(mol\right)\\ n_{H_2}=\dfrac{0,8}{2}=0,4\left(mol\right)\Rightarrow V_{H_2\left(đktc\right)}=0,4.22,4=8,96\left(l\right)\)

a) C2H5OH + O2 --men giấm--> CH3COOH + H2O
b) \(n_{O_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: C2H5OH + O2 --men giấm--> CH3COOH + H2O
0,3<----0,3------------------>0,3
=> \(m_{C_2H_5OH}=0,3.46=13,8\left(g\right)\)
=> \(V_{C_2H_5OH}=\dfrac{13,8}{0,8}=17,25\left(ml\right)\)
=> \(Độ.rượu=\dfrac{17,25}{150}.100=11,5^o\)
c) \(m_{CH_3COOH}=0,3.60=18\left(g\right)\)

\(n_{CO2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
Pt : \(C_6H_{12}O_6\xrightarrow[30-35^oC]{Menrượu}2C_2H_5OH+2CO_2\)
0,5 0,5
a) \(m_{C2H5OH}=0,5.46=23\left(g\right)\)
b) Pt : \(C_2H_5OH+O_2\xrightarrow[]{Mengiấm}CH_3COOH+H_2O\)
0,5 0,5
\(m_{CH3COOH\left(lt\right)}=0,5.60=30\left(g\right)\)
⇒ \(m_{CH3COOH\left(tt\right)}=30.80\%=24\left(g\right)\)
Chúc bạn học tốt
\(C_6H_{12}O_6\underrightarrow{t^o}2C_2H_5OH+2CO_2\uparrow\)(xt : men rượu )
0,5 0,5
\(n_{CO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
\(m_{C_2H_5OH}=0,5.46=23\left(g\right)\)
\(C_2H_5OH+O_2\underrightarrow{t^o}CH_3COOH+H_2O\) (men giấm )
0,5 0,5
\(m_{CH_3COOH}=0,5.60=30\left(g\right)\)
\(m_{CH_3COOHtt}=30.80\%=24\left(g\right)\)

a) $C_2H_5OH + 3O_2 \xrightarrow{t^o} 2CO_2 + 3H_2O$
b) $n_{C_2H_5OH} = \dfrac{9,2}{46} = 0,2(mol)$
Theo PTHH :
$n_{CO_2} = 2n_{C_2H_5OH} = 0,4(mol) \Rightarrow V_{CO_2} = 0,4.22,4 = 8,96(lít)$
$n_{H_2O} = 3n_{C_2H_5OH} = 0,6(mol) \Rightarrow m_{H_2O} = 0,6.18 = 10,8(gam)$
c) $CO_2 + Ca(OH)_2 \to CaCO_3 + H_2O$
$n_{CaCO_3} = n_{CO_2} = 0,4(mol)$
$m_{CaCO_3} = 0,4.100 = 40(gam)$

\(a.C_2H_5OH+3O_2-^{t^o}\rightarrow2CO_2+3H_2O\\ n_{C_2H_5OH}=0,3\left(mol\right)\\ n_{CO_2}=2n_{C_2H_5OH}=0,6\left(mol\right)\\ \Rightarrow V_{CO_2}=0,6.22,4=13,44\left(l\right)\\ b.n_{O_2}=3n_{C_2H_5OH}=0,6\left(mol\right)\\ MàV_{O_2}=\dfrac{1}{5}V_{kk}\\ \Rightarrow V_{kk}=V_{O_2}.5=0,6.22,4.5=67,2\left(l\right)\\ c.n_{NaOH}=0,9\left(mol\right)\\ Tacó:\dfrac{n_{NaOH}}{n_{CO_2}}=\dfrac{0,9}{0,6}=1,5\\ \Rightarrow Tạora2muốiNaHCO_3vàNa_2CO_3\\ Đặt:n_{NaHCO_3}=x\left(mol\right);n_{Na_2CO_3}=y\left(mol\right)\\ \Rightarrow\left\{{}\begin{matrix}x+y=0,6\left(BTnguyento\left(C\right)\right)\\x+2y=0,9\left(BTnguyento\left(Na\right)\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,3\\y=0,3\end{matrix}\right.\\ \Rightarrow m_{muối}=0,3.84+0,3.106=57\left(g\right)\)