Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(\left[O\right]_{KL}+H_2->H_2O\\ n_{H_2O}=n_{H_2}=\dfrac{14,4}{18}=0,8mol\\ v=0,8.22,4=17,92L\\ m_{KL}=m=47,2-16.0,8=34,4g\)

Bài 1:
a) PTHH: \(Fe_2O_3+3H_2\xrightarrow[]{t^o}2Fe+2H_2O\)
\(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
b) Ta có: \(\left\{{}\begin{matrix}m_{Fe_2O_3}=20\cdot80\%=16\left(g\right)\\m_{CuO}=20-16=4\left(g\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}n_{Fe_2O_3}=\dfrac{16}{160}=0,1\left(mol\right)\\n_{CuO}=\dfrac{4}{80}=0,05\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2}=3n_{Fe_2O_3}+n_{CuO}=0,35\left(mol\right)\) \(\Rightarrow V_{H_2}=0,35\cdot22,4=7,84\left(l\right)\)
c) Theo các PTHH: \(\left\{{}\begin{matrix}n_{Fe}=2n_{Fe_2O_3}=0,2\left(mol\right)\\n_{Cu}=n_{CuO}=0,05\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{hhB}=m_{Fe}+m_{Cu}=0,2\cdot56+0,05\cdot64=14,4\left(g\right)\)
Bài 2:
PTHH: \(Fe_2O_3+3H_2\xrightarrow[]{t^o}2Fe+3H_2O\)
\(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
a) Vì khối lượng Cu bằng \(\dfrac{6}{5}\) khối lượng Fe
\(\Rightarrow\left\{{}\begin{matrix}m_{Cu}=\dfrac{26,4}{6+5}\cdot6=14,4\left(g\right)\\m_{Fe}=26,4-14,4=12\left(g\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}n_{Cu}=\dfrac{14,4}{64}=0,225\left(mol\right)\\n_{Fe}=\dfrac{12}{56}=\dfrac{3}{14}\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{H_2}=\dfrac{3}{2}n_{Fe}+n_{Cu}=\dfrac{9}{28}+0,225=\dfrac{153}{280}\left(mol\right)\) \(\Rightarrow V_{H_2}=\dfrac{153}{280}\cdot22,4=12,24\left(l\right)\)
b) Theo các PTHH: \(\left\{{}\begin{matrix}n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=\dfrac{3}{28}\left(mol\right)\\n_{CuO}=n_{Cu}=0,225\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe_2O_3}=\dfrac{3}{28}\cdot160\approx17,14\left(g\right)\\m_{CuO}=0,225\cdot80=18\left(g\right)\end{matrix}\right.\) \(\Rightarrow m_{hh}=35,14\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe_2O_3}=\dfrac{17,14}{35,14}\cdot100\%\approx48,78\%\\\%m_{CuO}=51,22\%\end{matrix}\right.\)

4) x,y lần lượt là số mol của M và M2O3
=> nOxi=3y=nCO2=0,3 => y=0,1
Đề cho x=y=0,1 =>0,1M+0,1(2M+48)=21,6 =>M=56 => Fe và Fe2O3
=> m=0,1.56 + 0,1.2.56=16,8
2)X + 2HCl === XCl2 + H2
n_h2 = 0,4 => X = 9,6/0,4 = 24 (Mg)
=>V_HCl = 0,4.2/1 = 0,8 l

Ta có :
\(\dfrac{m_{FeO}}{m_{Fe_2O_3}}=\dfrac{9}{20}\Rightarrow\dfrac{72n_{FeO}}{160n_{Fe_2O_3}}=\dfrac{9}{20}\Rightarrow\dfrac{n_{FeO}}{n_{Fe_2O_3}}=\dfrac{9}{20}:\dfrac{72}{160}=1\)
Do đó, ta coi X chỉ gồm $Fe_3O_4$
$n_{Fe} = \dfrac{29,4}{56}= 0,525(mol)$
\(Fe_3O_4+4H_2\xrightarrow[]{t^o}3Fe+4H_2O\)
0,175 0,7 0,525 (mol)
$V = (0,7 : 80\%).22,4 = 19,6(lít)$
$m = (0,175 :80\%).232 = 50,75(gam)$

a) Gọi số mol H2 là x
=> \(n_{H_2O}=x\left(mol\right)\)
Theo ĐLBTKL: \(m_A+m_{H_2}=m_B+m_{H_2O}\)
=> 200 + 2x = 156 + 18x
=> x = 2,75 (mol)
=> \(V_{H_2}=2,75.22,4=61,6\left(l\right)\)
b) Gọi \(\left\{{}\begin{matrix}n_{CuO}=a\left(mol\right)\\n_{Fe_2O_3}=1,5a\left(mol\right)\\n_{Al_2O_3}=b\left(mol\right)\end{matrix}\right.\)
=> 80a + 240a + 102b = 200
=> 320a + 102b = 200
PTHH: CuO + H2 --to--> Cu + H2O
a---------------->a
Fe2O3 + 3H2 --to--> 2Fe + 3H2O
1,5a------------------>3a
=> 64a + 168a + 102b = 156
=> 232a + 102b = 156
=> a = 0,5; b = \(\dfrac{20}{51}\)
=> \(\left\{{}\begin{matrix}\%m_{CuO}=\dfrac{0,5.80}{200}.100\%=20\%\\\%m_{Fe_2O_3}=\dfrac{0,75.160}{200}.100\%=60\%\\\%m_{Al_2O_3}=\dfrac{\dfrac{20}{51}.102}{200}.100\%=20\%\end{matrix}\right.\)
c) \(n_{H_2}=\dfrac{2,75}{5}=0,55\left(mol\right)\)
\(n_{FeO\left(tt\right)}=\dfrac{36}{72}=0,5\left(mol\right)\)
Gọi số mol FeO phản ứng là t (mol)
PTHH: FeO + H2 --to--> Fe + H2O
t--------------->t
=> 56t + (0,5-t).72 = 29,6
=> t = 0,4 (mol)
=> \(H\%=\dfrac{0,4}{0,5}.100\%=80\%\)

\(n_{H_2}=\dfrac{5,04}{22,4}=0,225\left(mol\right)\)
PTHH: CuO + H2 → Cu + H2O
Mol: x x x
PTHH: Fe2O3 + 3H2 → 2Fe + 3H2O
Mol: y 3y 2y
Ta có hpt:\(\left\{{}\begin{matrix}80x+160y=14\\x+3y=0,225\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,075\left(mol\right)\\y=0,05\left(mol\right)\end{matrix}\right.\)
\(m_{hh.kim.loại}=m_{Cu}+m_{Fe}=0,075.64+2.0,05.56=10,4\left(g\right)\)
\(n_{H_2}=\dfrac{5,04}{22,4}=0,225\left(mol\right)\)
PTHH:
\(CuO+H_2\underrightarrow{t^o}Cu+H_2O\\ Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
Theo 2 pthh trên: \(n_{H_2O}=n_{H_2}=0,225\left(mol\right)\)
\(\rightarrow m_{H_2O}=0,225.18=4,05\left(g\right)\\ \rightarrow m_{H_2}=0,225.2=0,45\left(g\right)\)
Áp dụng ĐLBTKL, ta có:
\(m_{oxit\left(CuO,Fe_2O_3\right)}+m_{H_2}=m_{\text{kim loại}\left(Cu,Fe\right)}+m_{H_2O}\\ \rightarrow m_{\text{kim loại}\left(Cu,Fe\right)}=14+0,45-4,05=10,4\left(g\right)\)
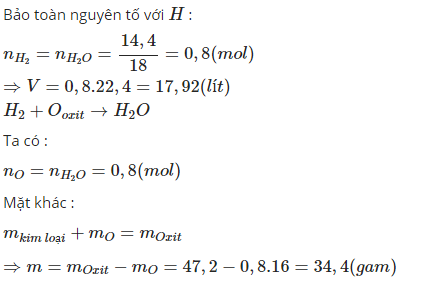
CuO+H2->Cu+H2O(1)
Fe2O3+3H2->2Fe+3H2O(2)
Fe3O4+4H2->3Fe+4H2O(3)
nH2O=0.8(mol)
Theo pthh(1)(2)(3) nH2O=nH2
->nH2 cần dùng=0.8(mol)
->V=0.8*22.4=17.92(l)
mH2=0.8*2=1.6(g)
Theo đlbtkl:mOxit+mH2=m nước+m kim loại
<->47.2+1.6=14.4+m kim loại
->m kim loại=47.2+1.6-14.4=34.4(g)
Cảm ơn bạn nhé