Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
a) Ta có: \(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)=n_{Zn}\)
\(\Rightarrow\%m_{Zn}=\dfrac{0,4\cdot65}{36,2}\cdot100\%\approx71,23\%\) \(\Rightarrow\%m_{Al_2O_3}=28,77\%\)
c) Ta có: \(n_{Al_2O_3}=\dfrac{36,2-0,4\cdot65}{102}=0,1\left(mol\right)\)
Theo PTHH: \(n_{HCl}=2n_{Zn}+6n_{Al_2O_3}=1,4\left(mol\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{1,4\cdot36,5}{10\%}=511\left(g\right)\) \(\Rightarrow V_{ddHCl}=\dfrac{511}{1,1}\approx464,5\left(ml\right)=0,4645\left(l\right)\)
c) Theo PTHH: \(\left\{{}\begin{matrix}n_{ZnCl_2}=0,4\left(mol\right)\\n_{AlCl_3}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{ZnCl_2}}=\dfrac{0,4}{0,4645}\approx0,86\left(M\right)\\C_{M_{AlCl_3}}=\dfrac{0,2}{0,4645}\approx0,43\left(M\right)\end{matrix}\right.\)

a.\(Fe+S\rightarrow\left(t^o\right)FeS\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(FeS+2HCl\rightarrow FeCl_2+H_2S\)
b.\(n_{hhk}=\dfrac{4,48}{22,4}=0,2mol\)
\(Fe+S\rightarrow\left(t^o\right)FeS\)
Ta thu được hh khí --> S hết, Fe dư
Gọi \(\left\{{}\begin{matrix}n_{Fe}=x\\n_S=y\end{matrix}\right.\)
\(\rightarrow n_{FeS}=n_{Fe}=n_S\rightarrow n_{Fe\left(dư\right)}=x-y\) ( mol )
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(x-y\) \(x-y\) ( mol )
\(FeS+2HCl\rightarrow FeCl_2+H_2S\)
y y ( mol )
Ta có: \(\left(x-y\right)+y=0,2\)
\(\Leftrightarrow x=0,2\)
Ta có:\(56x+32y=14,4\)
\(\Leftrightarrow56.0,2+32y=14,4\)
\(\Leftrightarrow y=0,1\)
\(\rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,2.56}{14,4}.100=77,77\%\\\%m_S=100\%-77,77\%=22,23\%\end{matrix}\right.\)

a)Fe + 2HCl ->FeCl2 + H2\(\uparrow\)
0.01 0.01
FeS + 2HCl ->FeCl2 + H2S\(\uparrow\)
0.1 0.1
H2S + Pb(NO3)2->PbS \(\downarrow\) + 2HNO3
0.1 0.1
nPbS =2.39/239=0.1 mol , n (hỗn hợp khí) =2.464/22.4=0.11 mol
n(H2)+n(H2S)=0.11 ->n(H2)=0.01 mol
V(H2)=n * 22.4 = 0.01*22.4=0.224(l)
V(H2S)=n*22.4=0.1*22.4=2.24(l)
m(Fe)=n*M=0.01*56=0.56(g)
m(FeS)=n*M=0.1*88=8.8(g)

a) Đặt \(\left\{{}\begin{matrix}n_{Mg}=a\left(mol\right)\\n_{Al}=b\left(mol\right)\end{matrix}\right.\) \(\Rightarrow24a+27b=5,1\) (1)
Ta có: \(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
Bảo toàn electron: \(2a+3b=0,5\) (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,1\cdot24}{5,1}\cdot100\%\approx47,06\%\\\%m_{Al}=52,94\%\end{matrix}\right.\)
b) Bảo toàn nguyên tố: \(n_{HCl}=2n_{H_2}=0,5\left(mol\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{0,5\cdot36,5}{7,3\%}=250\left(g\right)\)
\(\Rightarrow V_{HCl}=\dfrac{250}{1,2}\approx208,33\left(ml\right)\)

a) Gọi \(\left\{{}\begin{matrix}n_{Fe}=a\left(mol\right)\\n_{Zn}=b\left(mol\right)\end{matrix}\right.\) \(\Rightarrow56a+65b=12,1\) (1)
Ta có: \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Bảo toàn electron: \(2n_{Fe}+2n_{Zn}=2n_{H_2}\) \(\Rightarrow2a+2b=0,4\) (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,1\cdot56}{12,1}\cdot100\%\approx46,28\%\\\%m_{Zn}=53,72\%\end{matrix}\right.\)
b)
Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{FeSO_4}=n_{Fe}=n_{Zn}=n_{ZnSO_4}=0,1\left(mol\right)\\n_{H_2SO_4\left(p.ứ\right)}=n_{H_2}=0,2\left(mol\right)\Rightarrow\Sigma n_{H_2SO_4}=0,2\cdot110\%=0,22\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{FeSO_4}=0,1\cdot152=15,2\left(g\right)\\m_{ZnSO_4}=0,1\cdot161=16,1\left(g\right)\\m_{H_2}=0,2\cdot2=0,4\left(g\right)\\m_{H_2SO_4\left(dư\right)}=\left(0,22-0,2\right)\cdot98=1,96\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{KL}+m_{ddH_2SO_4}-m_{H_2}=211,7\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{FeSO_4}=\dfrac{15,2}{211,7}\cdot100\%\approx7,18\%\\C\%_{ZnSO_4}=\dfrac{16,1}{211,7}\cdot100\%\approx7,61\%\\C\%_{H_2SO_4}=\dfrac{1,96}{22,4}\cdot100\%\approx0,93\%\end{matrix}\right.\)
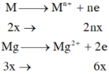
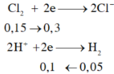
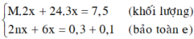

Chọn đáp án B
Khí thoát ra là N 2 không phản ứng.
% V c l o = 4 , 48 - 1 , 12 4 , 48 . 100 % =75%