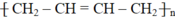Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

1. \(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
\(2C_2H_2\underrightarrow{t^o,p,xt}C_4H_4\)
\(CH_2=CH-C\equiv CH+H_2\underrightarrow{t^o,Pd}CH_2=CH-CH=CH_2\)
\(nCH_2=CH-CH=CH_2\underrightarrow{t^o,xt,p}\left(-CH_2-CH=CH-CH_2-\right)_n\)
2. \(CaC_2+2H_2O\rightarrow Ca\left(OH\right)_2+C_2H_2\)
\(CH\equiv CH+HCl\rightarrow CH_2=CHCl\)
\(nCH_2=CHCl\underrightarrow{t^o,p,xt}\left(-CH_2-CHCl-\right)_n\)
1. \(2CH_4\xrightarrow[1500^o]{làm.lạnh.nhanh}CH\equiv CH+3H_2\)
\(CH\equiv CH\xrightarrow[CuCl,NH_4Cl]{t^o}CH_2=CH-C\equiv CH\)
\(CH_2=CH-C\equiv CH+H_2\xrightarrow[Pd,PbCO_3]{t^o}CH_2=CH-CH=CH_2\)
\(nCH_2=CH-CH=CH_2\underrightarrow{t^o,xt,p}\left(-CH_2-CH=CH-CH_2-\right)n\)
2. \(CaC_2+2H_2O\rightarrow Ca\left(OH\right)_2+CH\equiv CH\)
\(CH\equiv CH+HCl\xrightarrow[HgCl_2]{t^o}CH_2=CHCl\)
\(nCH_2=CHCl\underrightarrow{t^o,xt,p}CH_2-CHCl\)

\(nCH_2=CH_2\rightarrow\left(-CH_2-CH_2-\right)_n\)
\(CH_4\underrightarrow{^{H2,1400oC}}C_2H_2\rightarrow H_2C=CH-C\equiv CH\underrightarrow{^{+H2}}H_2C=\)
\(CH-CH=CH_2\underrightarrow{^{to,xt}}-\left(-CH_2-CH=CH-CH_2-\right)_n-\)

\(Buta-1,3-dien :C_4H_6(x\ mol)\\ Penta-1,3-dien : C_5H_8(y\ mol)\\ \Rightarrow 54x + 68y = 21(1)\\ \)
Bảo toàn nguyên tố với H :
\(6x + 8y = 2n_{H_2O} = 2.\dfrac{21,6}{18} = 2,4(2)\\ (1)(2) \Rightarrow x = 0,2 ; y = 0,15\\ \Rightarrow m_{buta-1,3-dien} = 0,2.54 = 10,8\ gam\\ \Rightarrow m_{penta-1,3-dien} = 21 -10,8 = 10,2(gam)\)

a, \(CH_2=CH-CH=CH_2+Br_2\xrightarrow[1:1]{-80^oC}CH_2Br-CHBr-CH=CH_2\)
\(CH_2=CH-CH=CH_2+Br_2\xrightarrow[1:1]{40^oC}CH_2Br-CH=CH-CH_2Br\)
b, \(CH\equiv C-CH_3+H_2\xrightarrow[Pd/PbCO_3]{t^o}CH_2=CH-CH_3\)
c, \(3C_2H_2\xrightarrow[xt]{t^o,p}C_6H_6\)
d, \(CH\equiv CH+HCl\underrightarrow{HgCl_2}CH_2=CHCl\)
Bạn tham khảo nhé!

\(CaCO_3-^{t^o}\rightarrow CaO+CO_2\\ CaO+3C-^{t^o}\rightarrow CaC_2+CO\\ CaC_2+2H_2O\rightarrow C_2H_2+Ca\left(OH\right)_2\\2 C_2H_2-^{t^o,xt}\rightarrow CH\equiv C-CH=CH_2\\ CH\equiv C-CH=CH_2+H_2-^{t^o,Pd/PbCO_3}\rightarrow CH_2=CH-CH=CH_2\\ nCH_2=CH-CH=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2CH=CH-CH_2-\right)_n\)
\(a.nCH_2=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2-CH_2-\right)_n\\ b.nCH_2=C\left(CH_3\right)_2-^{t^o,p,xt}\rightarrow\left(-CH_2-C\left(CH_3\right)_2-\right)_n\\ c.nCH_2=CHCl-^{t^o,p,xt}\rightarrow\left(-CH_2-CHCl-\right)_n\\ d.nCH_2=CH-CH=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2CH=CH-CH_2-\right)_n\\ e.nCH_2-C\left(CH_3\right)-CH=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2-C\left(CH_3\right)=CH-CH_2-\right)_n\\ \)

Đáp án D
Các chất khi phản ứng hoàn toàn với H 2 dư (xúc tác Ni, đun nóng) tạo ra butan là: but-1-en; but-1-in; buta-1,3-đien; vinyl axetilen.

Đáp án A
Các chất thỏa mãn : axetilen, buta-1,3-dien, stiren, phenol, metyl acrylat.
=> Có 5 chất

Câu 1:
a. \(CH_2=CH-CH=CH_2+2H_2\underrightarrow{^{to,Ni}}CH_3-CH_2-CH_2-CH_3\)
b. \(CH_2=CH-CH=CH_2+Br_2\rightarrow Br-CH_2-CH\left(Br\right)-CH=CH_2\)
c. \(CH_2=CH-CH=CH_2+HBr\underrightarrow{^{40oC}}CH_3-CH=CH-CH_2-Br\)
d. \(CH_2=C\left(CH_3\right)-CH_2-CH_3+H_2\underrightarrow{^{Ni,to}}CH_3-C\left(CH_3\right)=CH-CH_3\)
e. \(CH_2=C\left(CH_3\right)-CH_2-CH_3+Br_2\rightarrow Br-CH_2-C\left(Br\right)\left(CH_3\right)-CH_2-CH_3\)
Câu 2:
- C2H2
\(CH\equiv CH\) : axelilen
- C3H4
\(CH\equiv C-CH_3\) : prop - 1 - in
- C4H6
\(CH\equiv C-CH_2-CH_3\) : but - 1 - in
\(CH_3-C\equiv C-CH_3\) : but - 2 -in
- C5H8
\(CH\equiv C-CH_2-CH_2-CH_3\) : pent - 1 - in
\(CH_3-C\equiv C-CH_2-CH_3\) : pent - 2 - in
\(CH\equiv C-CH\left(CH_3\right)-CH_3\) : 3 - metylbut - 1- in
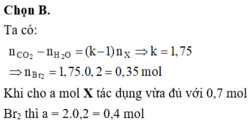
Đáp án B
Hướng dẫn Các phương trình phản ứng:
2CH4 CH
≡
CH + 3H2
CH
≡
CH + 3H2
CH2 = CH – C ≡ CH + H2 → P d CH2 = CH – CH = CH2
nCH2 = CH – CH = CH2 → x t