Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{O_2\left(đktc\right)}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ 4P+5O_2\underrightarrow{^{to}}2P_2O_5\\ 0,12........0,15.........0,06\left(mol\right)\\ m_P=0,12.31=3,72\left(g\right)\)

\(n_P=\dfrac{9.3}{31}=0.3\left(mol\right)\)
\(4P+5O_2\underrightarrow{^{^{t^0}}}2P_2O_5\)
\(0.3.....0.375.....0.15\)
\(V_{O_2}=0.375\cdot22.4=8.4\left(l\right)\)
\(m_{P_2O_5}=0.15\cdot142=21.3\left(g\right)\)
PT: 4P + 5O2 → 2P2O5.
Ta có: nP= 9,3/31=0,3(mol)
Theo PT: nO2= 5/4 . nP=5/4 . 0,3=0,375(mol)
=> VO2=0,375.22,4=8,4(lít)
Theo PT: nP2O5=1/2 . nP=1/2 . 0,3=0,15(mol)
=> mP2O5= 0,15.142=21,3(g)

PTHH: \(4P+5O_2\xrightarrow[]{t^o}2P_2O_5\)
Ta có: \(n_P=\dfrac{9,3}{31}=0,3\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{O_2}=0,375\left(mol\right)\\n_{P_2O_5}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{O_2}=0,375\cdot22,4=8,4\left(g\right)\\m_{P_2O_5}=0,15\cdot142=21,3\left(g\right)\end{matrix}\right.\)

a, Theo giả thiết ta có: \(n_P=\dfrac{3,1}{31}=0,1\left(mol\right)\)
\(4P+5O_2--t^o->2P_2O_5\)
Ta có: \(n_{O_2}=\dfrac{5}{4}.n_P=0,125\left(mol\right)\Rightarrow V_{O_2\left(đktc\right)}=0,125.22,4=2,8\left(l\right)\)
b, Theo giả thiết ta có: \(n_{CH_4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(CH_4+2O_2--t^o->CO_2+2H_2O\)
Ta có: \(n_{O_2}=2.n_{CH_4}=0,1\left(mol\right)\Rightarrow V_{O_2\left(đktc\right)}=2,24\left(l\right)\)

$\%m_{O_2(X)}=\dfrac{1,6}{1,6+4,4}.100\%=26,67\%$
$n_{CO_2}=\dfrac{4,4}{44}=0,1(mol);n_{O_2}=\dfrac{1,6}{16}=0,05(mol)$
$\Rightarrow \%V_{O_2(X)}=\dfrac{0,05}{0,05+0,1}.100\%=33,33\%$
$C+O_2\xrightarrow{t^o}CO_2$
Theo PT: $n_C=n_{O_2(p/ứ)}=n_{CO_2}=0,1(mol)$
$\Rightarrow n_{O_2(dùng)}=0,1+0,05=0,15(mol)$
$m_C=0,1.12=1,2(g);V_{O_2(dùng)}=0,15.22,4=3,36(lít)$
$\to m=1,2;V=3,36$

\(a)\\ n_{O_2} = \dfrac{6,72}{22,4} = 0,3(mol)\\ 4P + 5O_2 \xrightarrow{t^o} 2P_2O_5\\ \dfrac{n_P}{4} = 0,05 < \dfrac{n_{O_2}}{5} = 0,06\)
Do đó, Oxi dư.
\(n_{O_2\ pư} = \dfrac{5}{4}n_P = 0,25(mol)\\ \Rightarrow m_{O_2\ dư} = (0,3 - 0,25).32 = 1,6(gam)\\ b)\\ n_{P_2O_5} = \dfrac{n_P}{2} = 0,1(mol)\\ \Rightarrow m_{P_2O_5} = 0,1.142 = 14,2(gam)\)
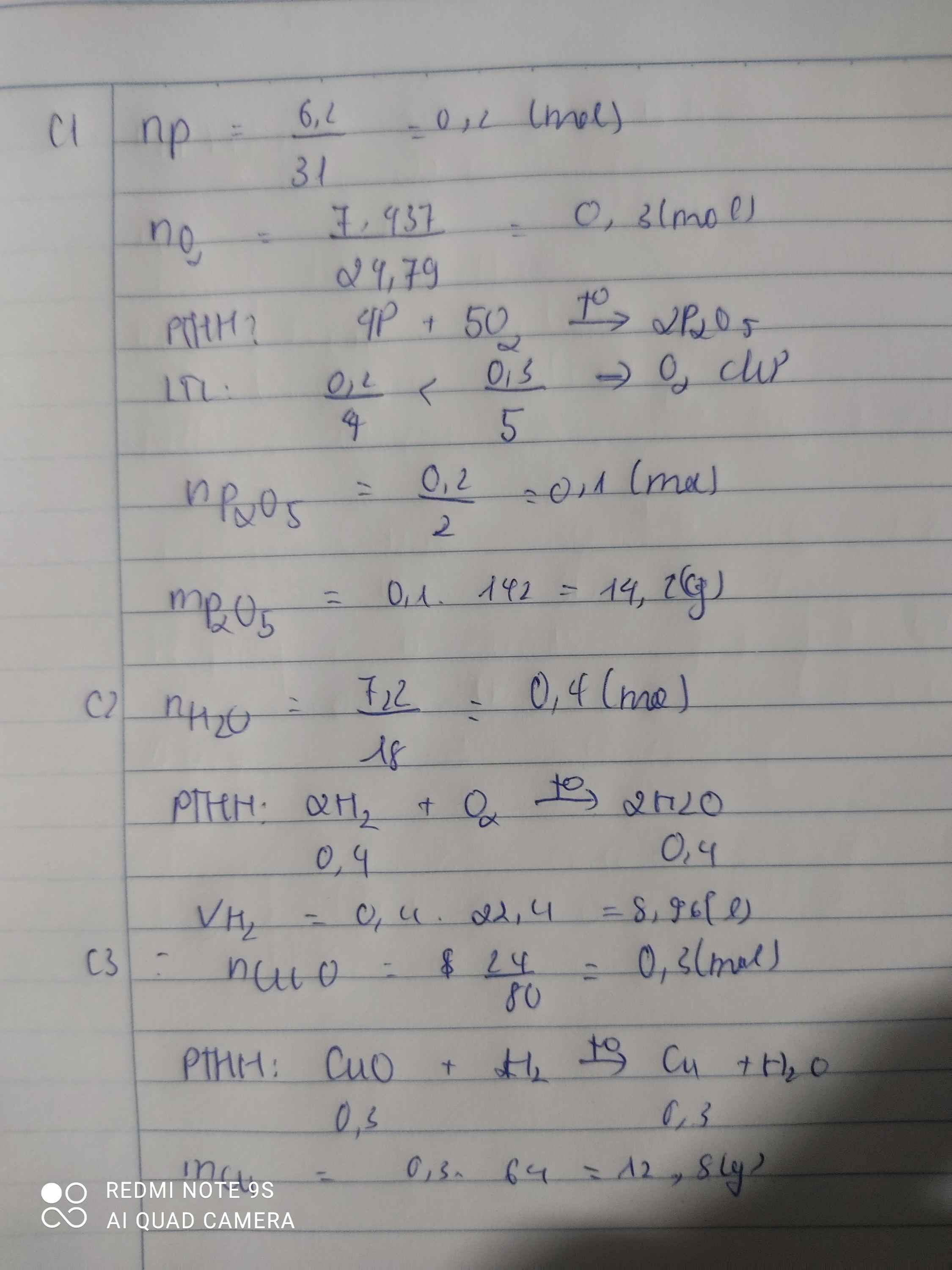
Câu 1:
\(4P+5O_2\rightarrow2P_2O_5\)
\(n_P=\dfrac{15.5}{31}=0.5\left(mol\right)\)
\(\Leftrightarrow n_{P_2O_5}=0.25\left(mol\right)\)
\(\Leftrightarrow m_{P_2O_5}=0.25\cdot142=35.5\left(g\right)\)
Câu 1:
4P + 5O2 \(\underrightarrow{t^o}\) 2P2O5
\(n_P=\dfrac{15,5}{31}=0,5mol\)
\(n_{O_2}=\dfrac{0,5.5}{4}=0,625mol\)
\(V_{O_2}=0,625.22,4=14l\)
\(n_{P_2O_5}=\dfrac{0,5.2}{4}=0,25mol\\ m_{P_2O_5}=0,25.142=35,5g\)
Câu 2:
4P + 5O2 \(\underrightarrow{t^o}\) 2P2O5
\(n_P=\dfrac{15,5}{31}=0,625mol\\ n_{P_2O_5}=\dfrac{0,625.2}{4}=0,25mol\\ m_{P_2O_5}=0,25.142=35,5g\)
\(Bài.2.có.nhiều.cách.làm.nhé.bạn\)