
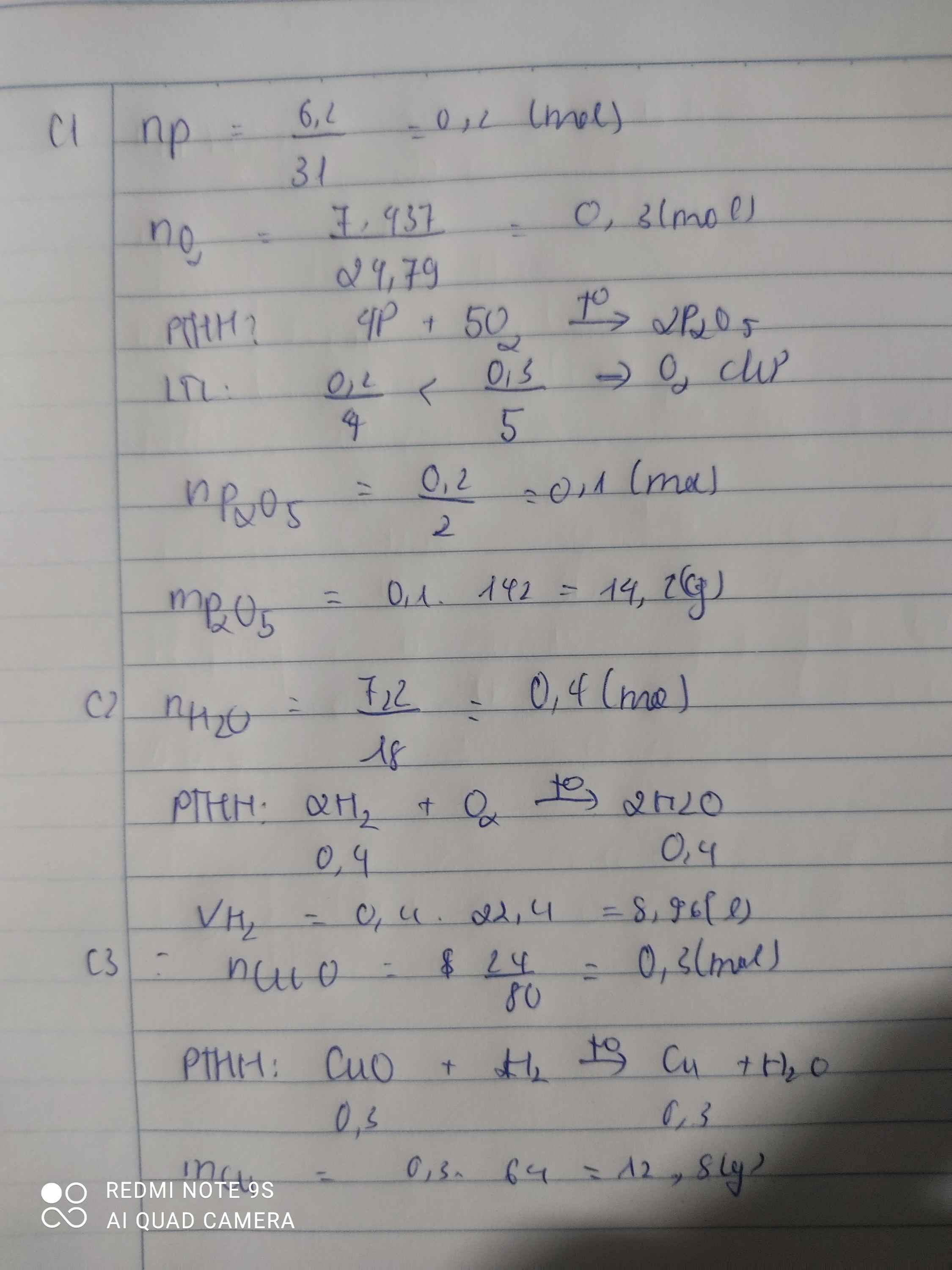
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

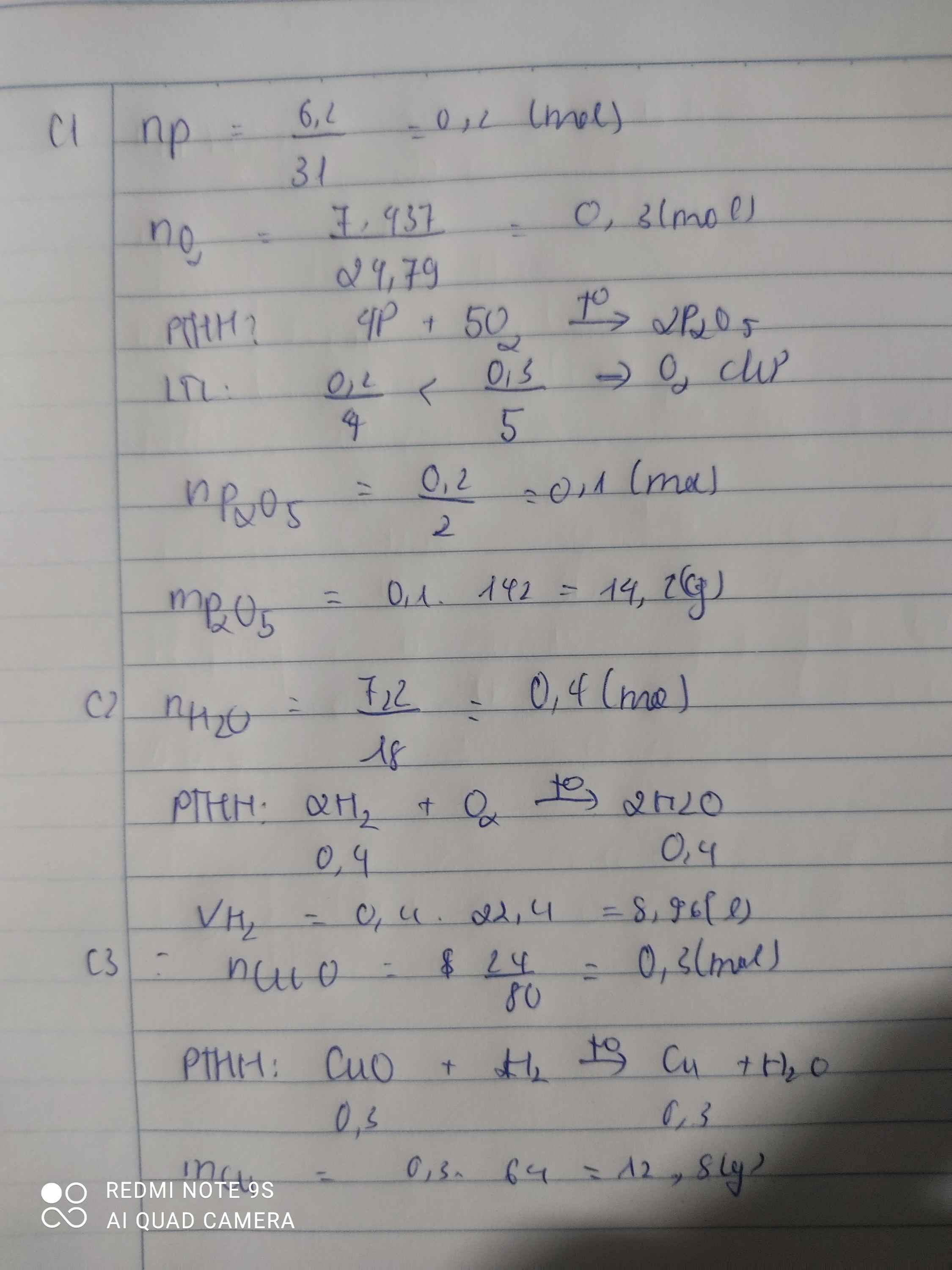

Ta có: \(n_P=\dfrac{12,4}{31}=0,4\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
_____0,4____0,5_____0,2 (mol)
a, \(m_{P_2O_5}=0,2.142=28,4\left(g\right)\)
b, \(V_{O_2}=0,5.22,4=11,2\left(l\right)\)

1.\(n_{Fe_3O_4}=\dfrac{2,32}{232}=0,01mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,3 0,1 ( mol )
\(m_{Fe}=0,3.56=16,8g\)
2.\(n_{Cu}=\dfrac{3,2}{64}=0,05mol\)
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\)
0,05 0,05 ( mol )
\(m_{CuO}=0,05.80=4g\)
3.\(n_{Na}=\dfrac{4,6}{23}=0,2mol\)
\(4Na+O_2\rightarrow\left(t^o\right)2Na_2O\)
0,2 0,05 ( mol )
\(V_{O_2}=0,05.24,79=1,2395l\)
4.\(n_{Cu}=\dfrac{1,6}{64}=0,025mol\)
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\)
0,025 0,0125 ( mol )
\(V_{O_2}=0,0125.24,79=0,309875l\)

a. \(n_{KMnO_4}=\dfrac{47.4}{158}=0,3\left(mol\right)\)
PTHH : 2KMnO4 ---to----> K2MnO4 + MnO2 + O2
0,3 0,15
\(V_{O_2}=0,15.22,4=3,36\left(l\right)\)
b. PTHH : 4Al + 3O2 -> 2Al2O3
0,2 0,15
\(m_{Al}=0,2.27=5,4\left(g\right)\)

Bài 2:
a) 2Mg + O2 --to--> 2MgO
b) \(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\)
PTHH: 2Mg + O2 --to--> 2MgO
_______0,2->0,1------>0,2
=> VO2 = \(\dfrac{0,1.0,082.\left(273+25\right)}{0,99}=2,468\left(l\right)\)
c) mMgO = 0,2.40 = 8(g)
Bài 3
a) Theo ĐLBTKL: mMg + mO2 = mMgO (1)
b) (1) => mMgO = 2,4 + 1,6 = 4(g)
c) \(nO_2=\dfrac{1,6}{32}=0,05\left(mol\right)\)
=> Số phân tử O2 = 0,05.6.1023 = 0,3.1023

Fe+2Hcl->FeCl2+H2
0,1---------------------0,1
2H2+O2-to>2H2O
0,1--------------0,1
n Fe=0,1 mol
=>VH2=0,1.22,4=2,24l
c) m H2O=0,1.18.95%=1,71g
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
PTHH:
Fe + 2HCl ---> FeCl2 + H2
0,1 0,1
2H2 + O2 --to--> 2H2O
0,1 0,1
\(\rightarrow\left\{{}\begin{matrix}V_{H_2}=0,1.22,4=2,24\left(l\right)\\m_{H_2O}=0,1.18.\left(100\%-5\%\right)=1,71\left(g\right)\end{matrix}\right.\)

\(a.2H_2+O_2-^{t^o}\rightarrow2H_2O\\ b.n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ n_{O_2}=\dfrac{1}{2}n_{H_2}=0,2\left(mol\right)\\ \Rightarrow V_{O_2}=0,2.22,4=4,48\left(l\right)\\ TrongkhôngkhíO_2chiếm20\%\\ \Rightarrow V_{kk}=\dfrac{4,48}{20\%}=22,4\left(l\right)\)

16 nCO2=0,2mol
PTHH: 2CO+O2=>2CO2
0,2<--0,1<---0,2
=> mO2=0,2.32=6,4g
=> khối lượng Oxi phản ứng với H2 là :
9,6-6,4=3,2g
=> nH2O=3,2:32=0,1mol
PTHH: 2H+O2=>H2O
b)
0,2<-0,1<-0,2
=> mH2=2.0,2=0,4g
mCO =0,2.28=5,6g
=> m hh=5,6+0,4=6g
CuO+H2-to--->Cu+H2O
0,6----0,6
nCuO =48/80=0,6 (mol)
==>VH2 =0,6×22,4=13.44(l)
17.
\(n_{Fe}=\dfrac{5,6}{56}=0,1mol\)
\(m_{H_2SO_4}=200.19,6\%=39,2g\)
\(n_{H_2SO_4}=\dfrac{39,2}{98}=0,4mol\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
0,1 < 0,4 ( mol )
0,1 0,1 0,1 0,1 ( mol )
\(V_{H_2}=0,1.22,4=2,24l\)
Chất còn dư là H2SO4
\(m_{H_2SO_4\left(dư\right)}=\left(0,4-0,1\right).98=29,4g\)
\(\left\{{}\begin{matrix}m_{FeSO_4}=0,1.152=15,2g\\m_{H_2}=0,1.2=0,2g\end{matrix}\right.\)
\(m_{ddspứ}=5,6+200-0,1.2=205,4g\)
\(\left\{{}\begin{matrix}C\%_{FeSO_4}=\dfrac{15,2}{205,4}.100=7,4\%\\C\%_{H_2}=\dfrac{0,2}{205,4}.100=0,09\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{29,4}{205,4}.100=14,31\%\end{matrix}\right.\)