Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
a_____2a______a_____a (mol)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\)
b_____3b_______b_____\(\dfrac{3}{2}\)b (mol)
Ta lập HPT: \(\left\{{}\begin{matrix}56a+27b=36,1\\a+\dfrac{3}{2}b=\dfrac{21,28}{22,4}=0,95\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,5\\b=0,3\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=0,5\cdot56=28\left(g\right)\\m_{Al}=8,1\left(g\right)\end{matrix}\right.\)
b+c) Theo các PTHH: \(\left\{{}\begin{matrix}n_{HCl}=2a+3b=1,9\left(mol\right)\\n_{FeCl_2}=0,5\left(mol\right)\\n_{AlCl_3}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{HCl}}=\dfrac{1,9}{0,2}=9,5\left(M\right)\\C_{M_{FeCl_2}}=\dfrac{0,5}{0,2}=2,5\left(M\right)\\C_{M_{AlCl_3}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\end{matrix}\right.\)

Câu 5 :
\(n_{H2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
a) Pt : \(Mg+2HCl\rightarrow MgCl_2+H_2|\)
1 2 1 1
0,1 0,2 0,1 0,1
\(MgO+2HCl\rightarrow MgCl_2+H_2O|\)
1 2 1 1
0,15 0,3 0,15
a) \(n_{Mg}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
\(m_{Mg}=0,1.24=2,4\left(g\right)\)
\(m_{MgO}=8,4-2,4=6\left(g\right)\)
0/0Mg = \(\dfrac{2,4.100}{8,4}=28,57\)0/0
0/0MgO = \(\dfrac{6.100}{8,4}=71,43\)0/0
b) Có : \(m_{MgO}=6\left(g\right)\)
\(n_{MgO}=\dfrac{6}{40}=0,15\left(mol\right)\)
\(n_{HCl\left(tổng\right)}=0,2+0,3=0,5\left(mol\right)\)
\(m_{HCl}=0,5.36,5=18,25\left(g\right)\)
\(m_{ddHCl}=\dfrac{18,25.100}{3,65}=500\left(g\right)\)
\(n_{MgCl2\left(tổng\right)}=0,1+0,15=0,25\left(mol\right)\)
⇒ \(m_{MgCl2}=0,15.95=14,25\left(g\right)\)
\(m_{ddspu}=8,4+500-\left(0,1.2\right)=508,2\left(g\right)\)
\(C_{MgCl2}=\dfrac{14,25.100}{508,2}=2,8\)0/0
Chúc bạn học tốt

\(n_{H_2}=\dfrac{6,1975}{24,79}=0,25(mol)\\ a,PTHH:Mg+2HCl\to MgCl_2+H_2\\ MgO+2HCl\to MgCl_2+H_2\\ b,n_{Mg}=n_{H_2}=0,25(mol)\\ \Rightarrow \%_{Mg}=\dfrac{0,25.24}{12}.100\%=50\%\\ \%_{MgO}=100\%-50\%=50\%\\ c,n_{MgO}=\dfrac{12-0,25.24}{40}=0,15(mol)\\ \Rightarrow \Sigma n_{HCl}=0,25.2+0,15.2=0,8(mol)\\ \Rightarrow x=C_{M_{HCl}}=\dfrac{0,8}{0,4}=2M\)
\(d,n_{MgCl_2}=0,25+0,15=0,4(mol)\\ \Rightarrow m_{MgCl_2}=0,4.95=38(g)\)

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(Ca+2HCl\rightarrow CaCl_2+H_2\)
0,1 0,1 ( mol )
\(\rightarrow\left\{{}\begin{matrix}\%m_{Ca}=\dfrac{0,1.40}{10}.100=40\%\\\%m_{MgO}=100\%-40\%=60\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}C\%_{CaCl_2}=\dfrac{0,1.111}{10+390,2-0,1.2}.100=2,775\%\\C\%_{MgO}=\dfrac{4}{10+390,2-0,1.2}.100=1\%\end{matrix}\right.\)

Câu 1:
a) CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
CaO + 2HCl --> CaCl2 + H2O
b)
\(n_{CO_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
_____0,4<---------0,8<-----------------0,4
=> mCaCO3 = 0,4.100 = 40(g)
=> mCaO = 62,4 - 40 = 22,4 (g)
c) \(n_{CaO}=\dfrac{22,4}{56}=0,4\left(mol\right)\)
CaO + 2HCl --> CaCl2 + H2O
_0,4-->0,8
=> nHCl = 0,8 + 0,8 = 1,6(mol)
=> \(C_{M\left(HCl\right)}=\dfrac{1,6}{0,25}=6,4M\)
Câu 1:
\(a,CaO+2HCl\to CaCl_2+H_2O\\ CaCO_3+2HCl\to CaCl_2+H_2O+CO_2\uparrow\\ b,n_{CO_2}=\dfrac{8,96}{22,4}=0,4(mol)\\ \Rightarrow n_{CaCO_3}=0,4(mol)\\ \Rightarrow m_{CaCO_3}=0,4.100=40(g)\\ \Rightarrow m_{CaO}=62,4-40=22,4(g)\\ c,n_{CaO}=\dfrac{22,4}{56}=0,4(mol)\\ \Rightarrow \Sigma n_{HCl}=0,4.2+0,4.2=1,6(mol)\\ \Rightarrow C_{M_{HCl}}=\dfrac{1,6}{0,25}=6,4M\)
Câu 2: Đề thiếu

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1=n_{Zn}\\ n_{ZnO}=\dfrac{14,6-6,5}{81}=0,1mol\\ C\%=\dfrac{0,2\cdot136}{175,6+14,6-0,2}=14,32\%\)

PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\) (1)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\) (2)
a) Ta có: \(n_{H_2}=\dfrac{22,4}{22,4}=1\left(mol\right)=n_{Mg}\) \(\Rightarrow m_{Mg}=1\cdot24=24\left(g\right)\)
\(\Rightarrow\%m_{Mg}=\dfrac{24}{32}\cdot100\%=75\%\) \(\Rightarrow\%m_{MgO}=25\%\)
b) Theo 2 PTHH: \(\left\{{}\begin{matrix}n_{HCl\left(1\right)}=2n_{Mg}=2mol\\n_{HCl\left(2\right)}=2n_{MgO}=2\cdot\dfrac{32-24}{40}=0,4mol\end{matrix}\right.\)
\(\Rightarrow\Sigma n_{HCl}=2,4mol\) \(\Rightarrow m_{ddHCl}=\dfrac{2,4\cdot36,5}{7,3\%}=1200\left(g\right)\)
c) Theo PTHH: \(\Sigma n_{MgCl_2}=\dfrac{1}{2}\Sigma n_{HCl}=1,2mol\)
\(\Rightarrow\Sigma m_{MgCl_2}=1,2\cdot95=114\left(g\right)\)
Mặt khác: \(m_{H_2}=1\cdot2=2\left(g\right)\)
\(\Rightarrow m_{dd}=m_{hh}+m_{ddHCl}-m_{H_2}=1230\left(g\right)\)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{114}{1230}\cdot100\%\approx9,27\%\)

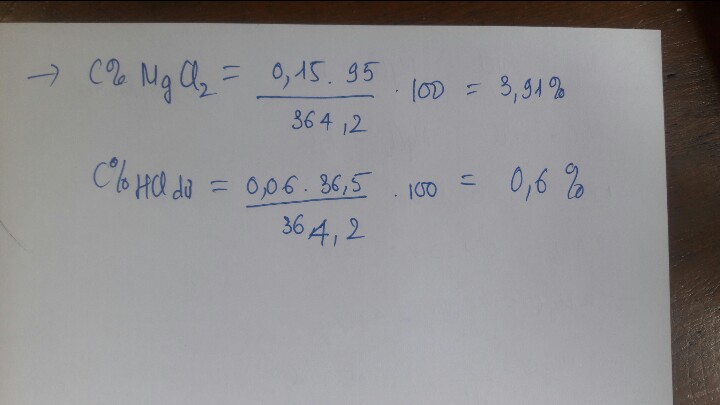
1) Số mol của CO2 là
nCO2 = \(\dfrac{6,72}{22,4}\)= 0,3 ( mol )
Pt : CaCO3 + 2HCl \(\rightarrow\) CaCl2 + CO2 + H2O ( 1)
MgO + 2HCl \(\rightarrow\) MgCl2 + H2O ( 2)
Số mol của CaCO3 là
nCaCO3 = nCo2 = 0,3 ( mol )
Khối lượng của CaCO3 là
mCaCO3 = 0,3 . 100 = 30g
Khối lượng của MgO là
mMgO = mhhX - mCaCO3 = 40 - 30 =10g
%CaCO3 = \(\dfrac{30}{40}\). 100% = 75%
%MgO = \(\dfrac{10}{40}\). 100% = 25%
2) Số mol HCl ở 2 PT ( 1) ,(2)
nHCl(1) = 2 nCaCO3 = 2 .0,3 = 0,6 ( mol )
nHCl (2) = 2nMgO = 2 .0,25 = 0,5 ( mol )
nHCl (1,2) = 0,6 + 0,5 = 1,1 ( mol )
Khối lượng của HCl ở 2 pt (1,2) là
mHCl = 1,1 . 36,5 = 40,15g
Khối lượng dd HCl là :
mdd HCl = \(\dfrac{40,15}{18,25}\). 100% = 220g
3) Dung dịch sau Phản ứng gồm : \(\left\{{}\begin{matrix}ddCaCl_2\\ddMgCl_2\end{matrix}\right.\)
nCaCl2 = nCaCO3 = 0,3( mol )
mCaCl2 = 0,3 . 111 = 33,3g
nMgCl2 = nMgO = 0,25 ( mol )
mMgCl2 = 0,25 . 95 = 23,75g
mdd sau phản ứng = mhhX + mddHCl - mCO2
= 40 + 220 - 13,2
= 246,8g
C%ddCaCl2 =\(\dfrac{33,3}{246,8}\).100% = 13,5%
C%ddMgCl2 = \(\dfrac{23,75}{246,8}\). 100% = 9,6%