Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
a.
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
b.
\(n_{Mg}=n_{H_2}=0,1\left(mol\right)\Rightarrow m_{Mg}=0,1.24=2,4\left(g\right),m_{CuO}=12-2,4=9,6\left(g\right)\)
c.
\(m_{muối}=m_{CuCl_2}+m_{MgCl_2}=0,12.135+95.0,1=25,7\left(g\right)\)

PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\) (1)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\) (2)
a) Ta có: \(n_{H_2}=\dfrac{22,4}{22,4}=1\left(mol\right)=n_{Mg}\) \(\Rightarrow m_{Mg}=1\cdot24=24\left(g\right)\)
\(\Rightarrow\%m_{Mg}=\dfrac{24}{32}\cdot100\%=75\%\) \(\Rightarrow\%m_{MgO}=25\%\)
b) Theo 2 PTHH: \(\left\{{}\begin{matrix}n_{HCl\left(1\right)}=2n_{Mg}=2mol\\n_{HCl\left(2\right)}=2n_{MgO}=2\cdot\dfrac{32-24}{40}=0,4mol\end{matrix}\right.\)
\(\Rightarrow\Sigma n_{HCl}=2,4mol\) \(\Rightarrow m_{ddHCl}=\dfrac{2,4\cdot36,5}{7,3\%}=1200\left(g\right)\)
c) Theo PTHH: \(\Sigma n_{MgCl_2}=\dfrac{1}{2}\Sigma n_{HCl}=1,2mol\)
\(\Rightarrow\Sigma m_{MgCl_2}=1,2\cdot95=114\left(g\right)\)
Mặt khác: \(m_{H_2}=1\cdot2=2\left(g\right)\)
\(\Rightarrow m_{dd}=m_{hh}+m_{ddHCl}-m_{H_2}=1230\left(g\right)\)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{114}{1230}\cdot100\%\approx9,27\%\)

a) Gọi $n_{Al} = a(mol) ; n_{Fe} = b(mol) \Rightarrow 27a + 56b = 33,4(1)$
$2Al + 6HCl \to 2AlCl_3 + 3H_2$
$Fe + 2HCl \to FeCl_2 + H_2$
Theo PTHH : $n_{H_2} = 1,5a + b = \dfrac{17,92}{22,4} = 0,8(2)$
Từ (1)(2) suy ra : a = 0,2 ; b = 0,5
$\%m_{Al} = \dfrac{0,2.27}{33,4}.100\% = 16,17\%$
$\%m_{Fe} = 100\% - 16,17\% = 83,83\%$
b) $n_{HCl} = 2n_{H_2} = 1,6(mol)$
c) $m_{muối} = m_{hh} + m_{HCl} - m_{H_2} = 33,4 + 1,6.36,5 - 0,8.2 = 90,2(gam)$

nH2 \(\approx\)0,2 (mol)
Mg + 2HCl \(\rightarrow\) MgCl2 + H2 (1)
0,2 <------------ 0,2 <----- 0,2 (mol)
MgO + 2HCl \(\rightarrow\) MgCl2 + H2O (2)
b) %mMg = \(\frac{0,2.24}{8,8}\) . 100% =54,55%
%mMgO = 45,45%
c) mMgO = 8,8 - 0,2 . 24 = 4(g)
=> nMgO=0,1 (mol)
Theo pt(2) nMgCl2 = nMg = 0,1 (mol)
=> \(\Sigma n_{MgCl_2}\) = 0,2 + 0,1 = 0,3 (mol)
mmuối = 0,3 . 95 = 28,5 (g)

a) nH2=0,2(mol)
PTHH: Mg + 2 HCl -> MgCl2 + H2
0,2______0,4_______0,2_____0,2(mol)
PTHH: MgO +2 HCl -> MgCl2 + H2O
b) mMgO= 8,8 - 0,2.24=4(g)
%mMgO= (4/8,8).100= 45,455%
=>%Mg=54,545%
c) nHCl(tổng)= 2. nMg + 2. nMgO= 2. 0,2+ 0,1.2=0,6(mol)
=> mHCl= 0,6.36,5=21,9(g)
=>mddHCl=(21,9.100)/7,3=300(g)
d) mddMgCl2= mddHCl + m(hỗn hợp ban đầu) - mH2
<=>mddHCl= 300+ 8,8- 0,2.2= 308,4(g)
nMgCl2=0,3(mol) => mMgCl2= 0,3.95=28,5(g)
=>C%ddMgCl2= (28,5/308,4).100=9,241%

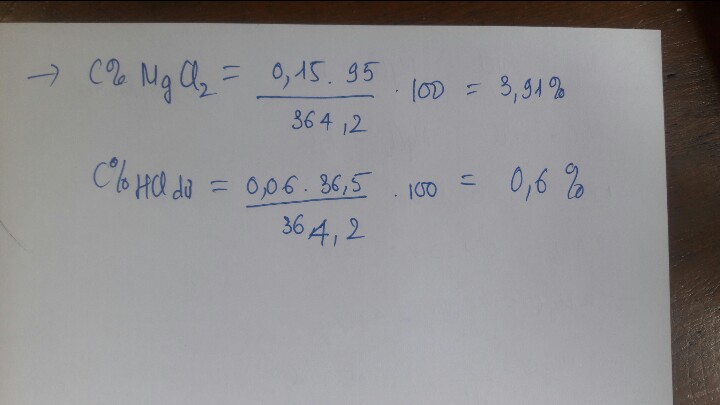
\(n_{HCl}=0,4.0,2=0,08\left(mol\right)\\PTHH:\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
x------->2x----->x
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
y------->2y------>y
Có hệ PT: \(\left\{{}\begin{matrix}80x+40y=2,8\\2x+2y=0,08\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,03\\y=0,01\end{matrix}\right.\)
a)
\(m_{CuO}=80.0,03=2,4\left(g\right)\\ m_{MgO}=40.0,01=0,4\left(g\right)\)
b)
\(\%_{m_{CuO}}=\dfrac{2,4.100\%}{2,8}=85,71\%\\ \%_{m_{MgO}}=\dfrac{0,4.100\%}{2,8}=14,29\%\)
c)
\(m_{CuCl_2}=135x=135.0,03=4,05\left(g\right)\)