Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

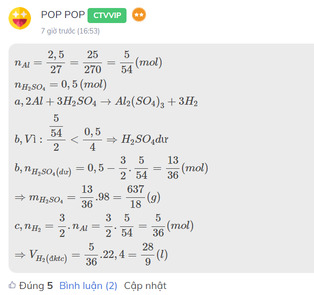
\(n_{Al}=\dfrac{2,5}{27}=\dfrac{25}{270}=\dfrac{5}{54}\left(mol\right)\\ n_{H_2SO_4}=0,5\left(mol\right)\\ a,2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ b,Vì:\dfrac{\dfrac{5}{54}}{2}< \dfrac{0,5}{4}\Rightarrow H_2SO_4dư\\ b,n_{H_2SO_4\left(dư\right)}=0,5-\dfrac{3}{2}.\dfrac{5}{54}=\dfrac{13}{36}\left(mol\right)\\ \Rightarrow m_{H_2SO_4}=\dfrac{13}{36}.98=\dfrac{637}{18}\left(g\right)\\ c,n_{H_2}=\dfrac{3}{2}.n_{Al}=\dfrac{3}{2}.\dfrac{5}{54}=\dfrac{5}{36}\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=\dfrac{5}{36}.22,4=\dfrac{28}{9}\left(l\right)\)

a: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
b: \(n_{Al}=\dfrac{2.5}{27}< \dfrac{1}{4}\)
=>H2SO4 dư, Al đủ
\(m_{H_2SO_4}=0.25\cdot98=24.5\left(g\right)\)
c: \(n_{Al_2\left(SO_4\right)_3}=\dfrac{2.5}{54}=\dfrac{5}{108}\left(mol\right)\)
\(\Leftrightarrow n_{H_2}=\dfrac{5}{36}\left(mol\right)\)
\(V_{H_2}=\dfrac{5}{36}\cdot22.4=\dfrac{28}{9}\left(lít\right)\)
Mình thấy bạn Thịnh tính lượng dư sai
Đây là bài mình từng làm, bạn tham khảo nhé!

PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
Làm gộp các phần còn lại
Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\) \(\Rightarrow\left\{{}\begin{matrix}n_{Al_2\left(SO_4\right)_3}=0,1mol\\n_{H_2SO_4}=n_{H_2}=0,3mol\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}V_{H_2}=0,3\cdot22,4=6,72\left(l\right)\\m_{Al_2\left(SO_4\right)_3}=0,1\cdot342=34,2\left(g\right)\\m_{H_2SO_4}=0,3\cdot98=29,4\left(g\right)\end{matrix}\right.\)

a, PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow V_{H_2}=0,2.22,4=4,48\left(l\right)\)
b, \(n_{Fe_2O_3}=\dfrac{6,4}{160}=0,04\left(mol\right)\)
PT: \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
Xét tỉ lệ: \(\dfrac{0,04}{1}< \dfrac{0,2}{3}\), ta được H2 dư.
Theo PT: \(n_{H_2\left(pư\right)}=3n_{Fe_2O_3}=0,12\left(mol\right)\Rightarrow n_{H_2\left(dư\right)}=0,2-0,12=0,08\left(mol\right)\)
\(\Rightarrow m_{H_2\left(dư\right)}=0,08.2=0,16\left(g\right)\)
Theo PT: \(n_{Fe}=2n_{Fe_2O_3}=0,08\left(mol\right)\Rightarrow m_{Fe}=0,08.56=4,48\left(g\right)\)

a) $n_{Al} = \dfrac{0,81}{27} = 0,03(mol) ; n_{HCl} = \dfrac{1,825}{36,5} = 0,05(mol)$
$2Al + 6HCl \to 2AlCl_3 + 3H_2$
Vì :
$n_{Al} : 2 > n_{HCl} : 6$ nên Al dư
$n_{H_2} = \dfrac{1}{2}n_{HCl} = 0,025(mol)$
$V_{H_2} = 0,025.24,79 = 0,61975(lít)$
b) $n_{Al\ pư} = \dfrac{1}{3} n_{HCl} = \dfrac{0,05}{3}(mol)$
Ta thấy : $m_{Al} - m_{H_2} = \dfrac{0,05}{3}.27 - 0,025.2 = 0,4 > 0$
Do đó, dung dịch tăng so với khối lượng dung dịch HCl ban đầu 0,4 gam

nP=\(\dfrac{62}{31}\)=0,2(mol)
nO2=\(\dfrac{7,84}{22,4}\)=0,35(mol)
PTHH:4P+5O2to→2P2O5
tpứ: 0,2 0,35
pứ: 0,2 0,25 0,1
spứ: 0 0,1 0,1
a)chất còn dư là oxi
mO2dư=0,1.32=3,2(g)
b)mP2O5=n.M=0,1.142=14,2(g)
\(a.n_P=0,2\left(mol\right);n_{O_2}=0,35\left(mol\right)\\ 4P+5O_2-^{t^o}\rightarrow2P_2O_5\\ LTL:\dfrac{0,2}{4}< \dfrac{0,35}{5}\\ \Rightarrow SauphảnứngO_2dư\\ n_{O_2\left(pứ\right)}=\dfrac{5}{4}n_P=0,25\left(mol\right)\\ \Rightarrow m_{P\left(dư\right)}=\left(0,35-0,25\right).32=3,2\left(g\right)\\ b.n_{P_2O_5}=\dfrac{1}{2}n_P=0,1\left(mol\right)\\ \Rightarrow m_{P_2O_5}=0,1.142=14,2\left(g\right)\)

PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
Ta có: \(\left\{{}\begin{matrix}n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\n_{HCl}=0,25\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,25}{2}\) \(\Rightarrow\) HCl còn dư, Kẽm p/ứ hết
\(\Rightarrow\left\{{}\begin{matrix}n_{H_2}=0,1\left(mol\right)\\n_{HCl\left(dư\right)}=0,05\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{H_2}=22,4\cdot0,1=2,24\left(l\right)\\m_{HCl\left(dư\right)}=0,05\cdot36,5=1,825\left(g\right)\end{matrix}\right.\)

mFe= 8,4/56= 0,15 mol
m HCl = 14,6/36,5=0,4 mol
PTHH: Fe +2HCl →FeCl2 +H2
Bđ: 0,15 0,4 0 0 mol
Pứ: o,15→0,3 0,15 0,15 mol
Sau pứ:0 0,1 0,15 0,15 mol
a. HCl dư: m =0,1.36,5=3,65 g
b. m FeCl2 = 0,15.127=19,05 g
c. m H2 = 0,15.2= 0,3 g
V H2= 0,15.22,4=3,36 (l)
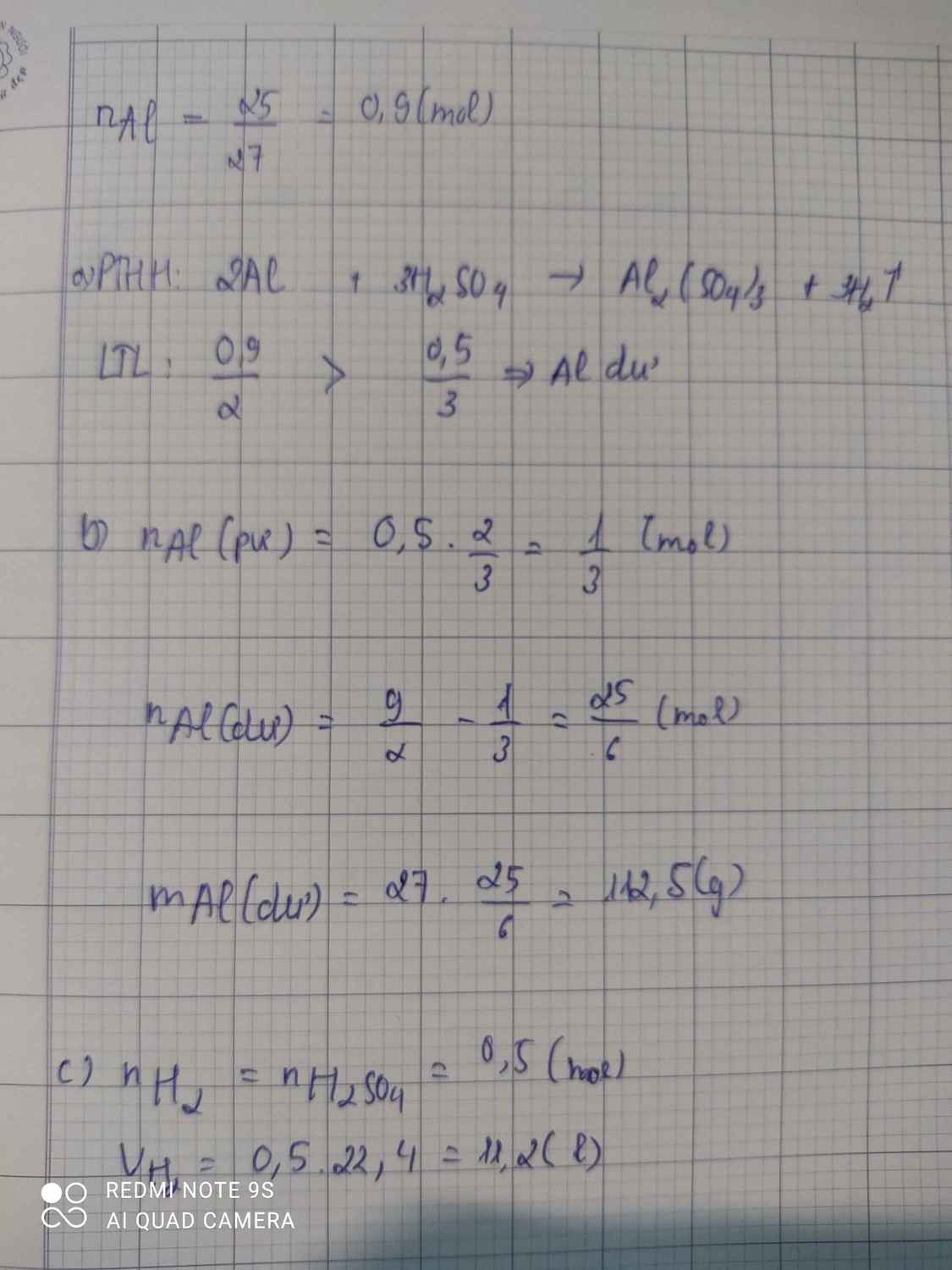
a, PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
Ta có: \(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{2}>\dfrac{0,25}{6}\), ta được Al dư.
Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{HCl}=0,125\left(mol\right)\Rightarrow V_{H_2}=0,125.22,4=2,8\left(l\right)\)
b, Theo PT: \(n_{Al\left(pư\right)}=\dfrac{1}{3}n_{HCl}=\dfrac{1}{12}\left(mol\right)\Rightarrow n_{Al\left(dư\right)}=0,1-\dfrac{1}{12}=\dfrac{1}{60}\left(mol\right)\)
\(\Rightarrow m_{Al}=\dfrac{1}{60}.27=0,45\left(g\right)\)