Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Zn}=\dfrac{8,125}{65}=0,125mol\)
\(n_{HCl}=\dfrac{18,25}{36,5}=0,5mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Xét: \(\dfrac{0,125}{1}\) < \(\dfrac{0,5}{2}\) ( mol )
0,125 0,125 ( mol )
\(V_{H_2}=0,125.22,4=2,8l\)

\(1.n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\\ PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
2. Theo PT, ta có: \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\)
\(V_{H_2}=0,2.22,4=4,48\left(l\right)\)

Zn+2H2SO4->ZnSO4+H2
0,2----------------------------0,2
n Zn=\(\dfrac{13}{65}\)=0,2 mol
=>VH2=0,2.22,4=4,48l
b)
Fe+2HCl->FeCl2+H2
0,1--------------0,05 mol
=>VH2=0,05.22,4=1,12l
\(a,n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\\ Zn+H_2SO_4\rightarrow ZnSO_4+H_2\\ n_{H_2}=n_{Zn}=0,2\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\\ b,Fe+2HCl\rightarrow FeCl_2+H_2\\ n_{HCl}=0,1\left(mol\right)\Rightarrow n_{H_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\)

PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
Ta có: \(n_{Zn}=\dfrac{32,5}{65}=0,5\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{HCl}=1\left(mol\right)\\n_{H_2}=0,5\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{ddHCl}=\dfrac{1\cdot36,5}{20\%}=182,5\left(g\right)\\V_{H_2}=0,5\cdot22,4=11,2\left(l\right)\end{matrix}\right.\)

\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{49}{98}=0,5\left(mol\right)\)
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
Xét tỉ lệ: \(\dfrac{0,2}{2}< \dfrac{0,5}{3}\) => Al hết, H2SO4 dư
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
0,2---------------------------->0,3
=> VH2 = 0,3.22,4 = 6,72 (l)
\(n_{Al}=\dfrac{m}{M}=\dfrac{5,4}{27}=0,2=\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{m}{M}=\dfrac{49}{98}=0,5\left(mol\right)\)
2Al + 3H2SO4 → Al2(SO4)3 + 3H2
2 3 ( mol )
0,2 0,5 ( mol )
Tỉ lệ: \(\dfrac{0,2}{2}< \dfrac{0,5}{3}\) ⇒ H2SO4 dư
2Al + 3H2SO4 → Al2(SO4)3 + 3H2
0,2 → 0,3 → 0,3 ( mol )\(V_{H_2}=n.22,4=0,3.22,4=6,72\left(l\right)\)
Zn+2HCl->ZnCl2+H2
0,2-----0,4---0,2----0,2
nZn=0,2 mol
=>m Hcl=0,4.36,5=14,6g
m muối=0,2.136=27,2g
=>VH2=0,2.22,4=4,48l
`Zn + 2HCl -> ZnCl_2 + H_2` `\uparrow`
`n_(Zn) = 13/65 = 0,2 mol`.
`n_(HCl) = 0,4 mol`.
`m_(HCl) = 0,4 xx 36,5 = 14,6g`.
c, `m_(ZnCl_2) = 0,2 xx 127 = 25,4 g`.
`d, V_(H_2) = 0,2 xx 22,4 = 4,48l`.
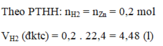
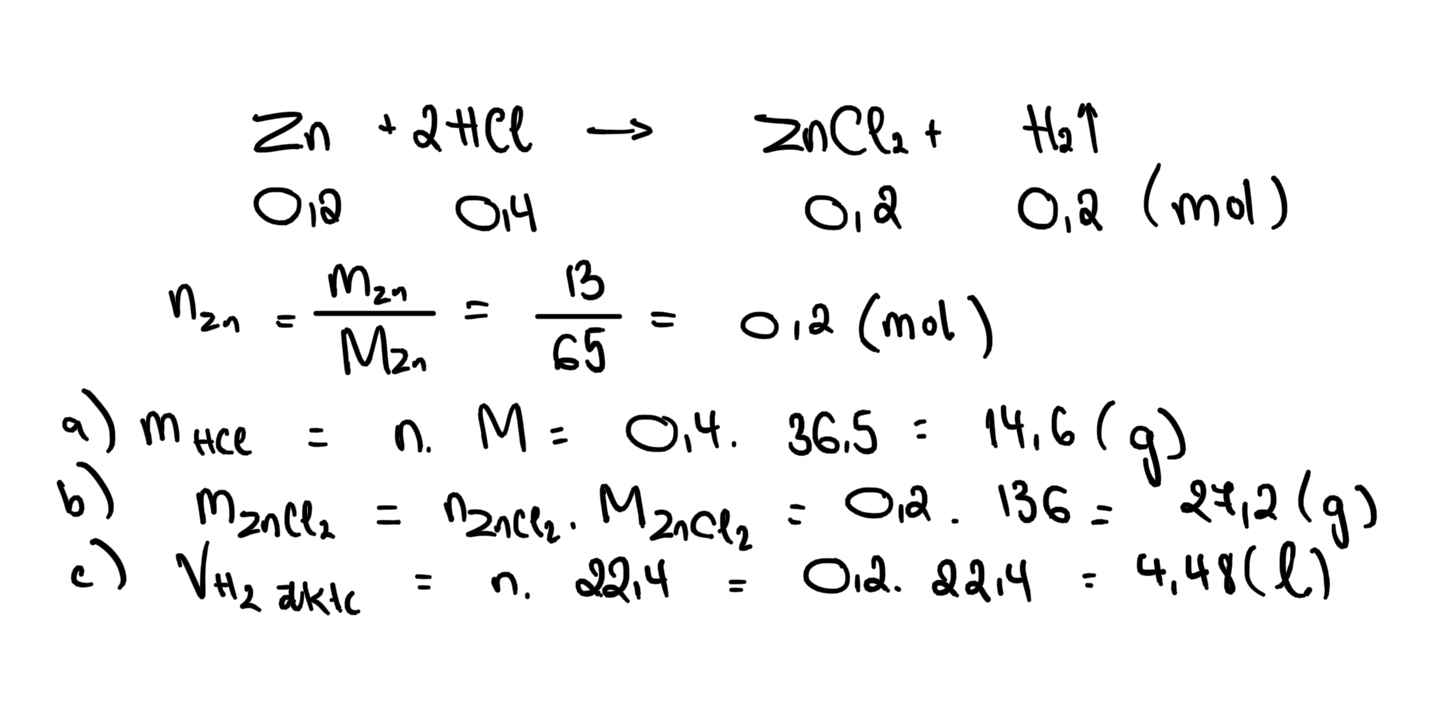
\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\); \(n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
Xét tỉ lệ: \(\dfrac{0,2}{1}=\dfrac{0,4}{2}\) => pư vừa đủ
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2----------------->0,2
=> VH2 = 0,2.22,4 = 4,48 (l)
nZn=13/65=0,2mol
nHCl=14,6/36,5=0,4mol
Zn+2HCl→ZnCl2+H2
Xét: 0,2 < 0,4 ( mol )
0,4 0,4 ( mol )
VH2=0,4.22,4=8,96