Giúp mik với ạ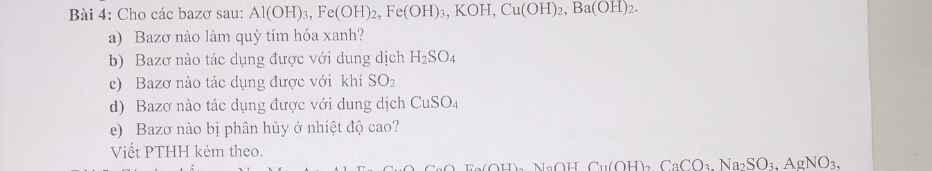
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Câu 3:
a: \(BD=\sqrt{BC^2-DC^2}=4\left(cm\right)\)
b: \(\widehat{A}=180^0-2\cdot70^0=40^0< \widehat{B}\)
nên BC<AC=AB
c: Xét ΔEBC vuông tại E và ΔDCB vuông tại D có
BC chung
\(\widehat{EBC}=\widehat{DCB}\)
Do đó:ΔEBC=ΔDCB
d: Xét ΔOBC có \(\widehat{OBC}=\widehat{OCB}\)
nên ΔOBC cân tại O
Câu 2
a) Thay y = -2 vào biểu thức đã cho ta được:
2.(-2) + 3 = -1
Vậy giá trị của biểu thức đã cho tại y = -2 là -1
b) Thay x = -5 vào biểu thức đã cho ta được:
2.[(-5)² - 5] = 2.(25 - 5) = 2.20 = 40
Vậy giá trị của biểu thức đã cho tại x = -5 là 40


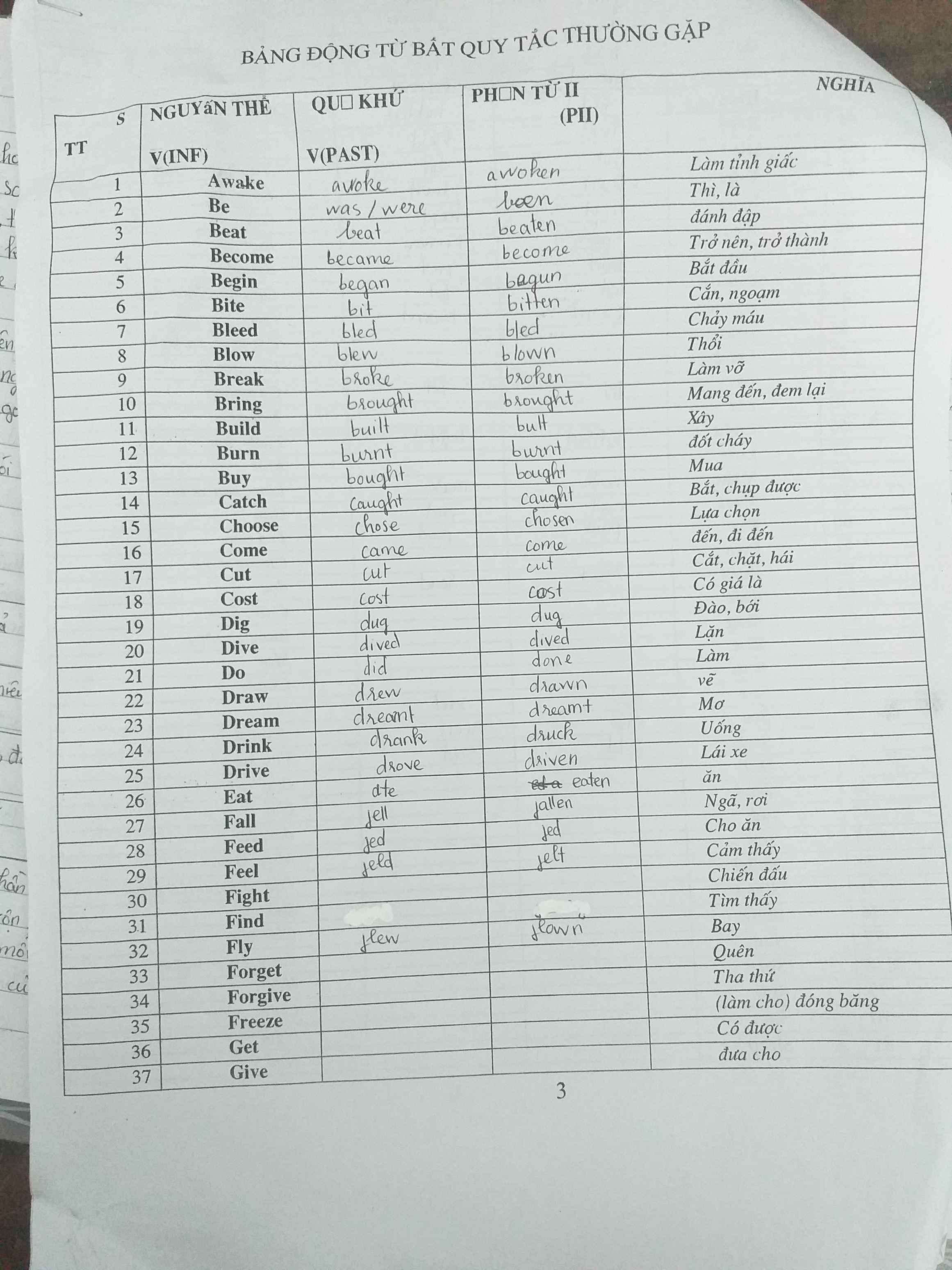
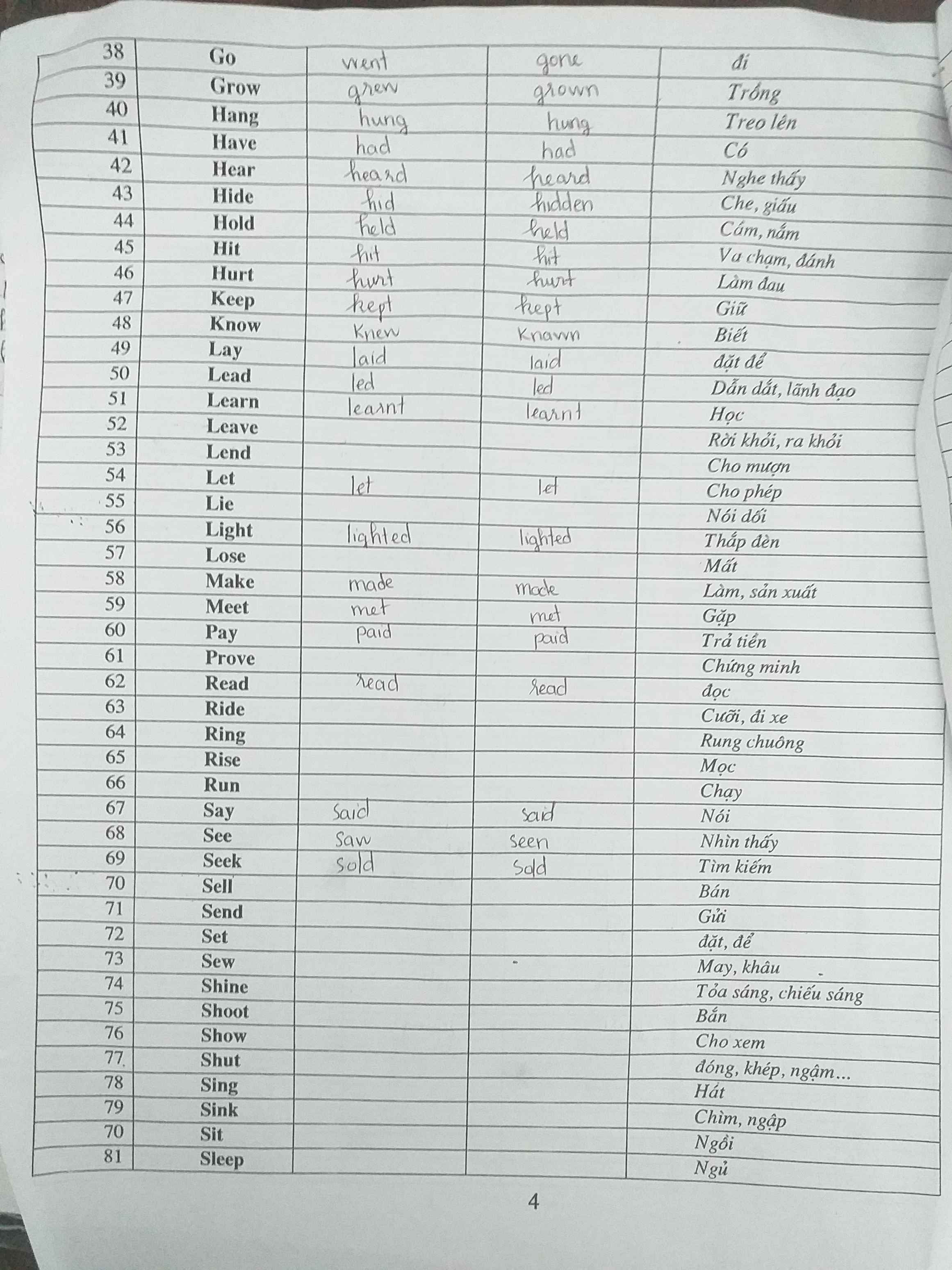
1 were - would you play
2 weren't studying - would have
3 had taken - wouldn't have got
4 would you go - could
5 will you give - is
6 recycle - won't be
7 had heard - wouldn't have gone
8 would you buy - had
9 don't hurry - will miss
10 had phoned - would have given
11 were - wouldn't eat
12 will go - rains
13 had known - would have sent
14 won't feel - swims
15 hadn't freezed - would have gone

\(a,=\dfrac{x^3+2x}{\left(x-1\right)\left(x^2+x+1\right)}+\dfrac{2}{x^2+x+1}-\dfrac{1}{x-1}=\dfrac{x^3+2x+2x-2-\left(x^2+x+1\right)}{\left(x-1\right)\left(x^2+x+1\right)}=\dfrac{x^3+3x-3}{\left(x-1\right)\left(x^2+x+1\right)}=\dfrac{x^3+3}{\left(x^2+x+1\right)}\)

a: \(=\dfrac{x^3+2x+2x-2-x^2-x-1}{\left(x-1\right)\left(x^2+x+1\right)}\)
\(=\dfrac{x^3-x^2+3x-3}{\left(x-1\right)\left(x^2+x+1\right)}=\dfrac{x^2+3}{x^2+x+1}\)
b: \(=\dfrac{x^2-2x-3+x^2+2x-3+2x-2x^2}{\left(x-3\right)\left(x+3\right)}\)
\(=\dfrac{2x-6}{\left(x-3\right)\left(x+3\right)}=\dfrac{2}{x+3}\)
c: \(=\dfrac{6-7+x}{3\left(x-1\right)}=\dfrac{x-1}{3\left(x-1\right)}=\dfrac{1}{3}\)
d: \(=\dfrac{x^3+2x+2x-2-x^2-x-1}{\left(x-1\right)\left(x^2+x+1\right)}=\dfrac{x^3-x^2+3x-3}{\left(x-1\right)\left(x^2+x+1\right)}=\dfrac{x^2+3}{x^2+x+1}\)

1 is explained
2 was stolen
3 will be opened
4 is being closed
5 is going to be built

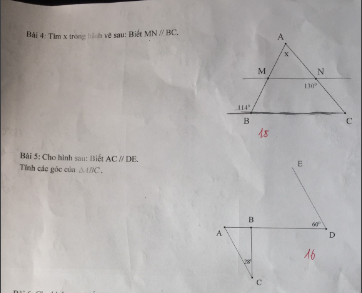 giúp mik với ạ mik cần gấp. GIẢI CỤ THỂ GIÚP MIK vs ạ
giúp mik với ạ mik cần gấp. GIẢI CỤ THỂ GIÚP MIK vs ạ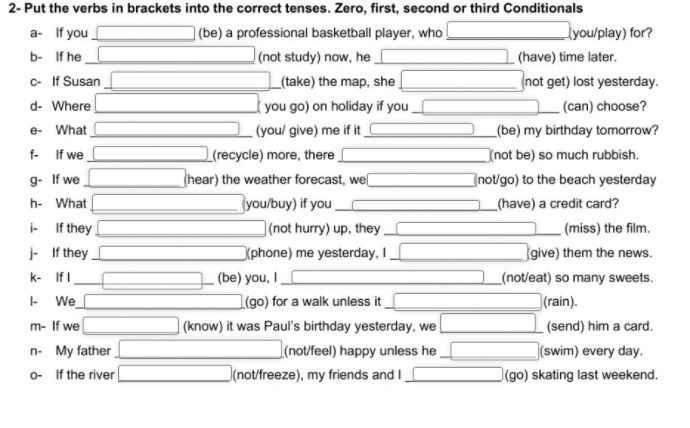
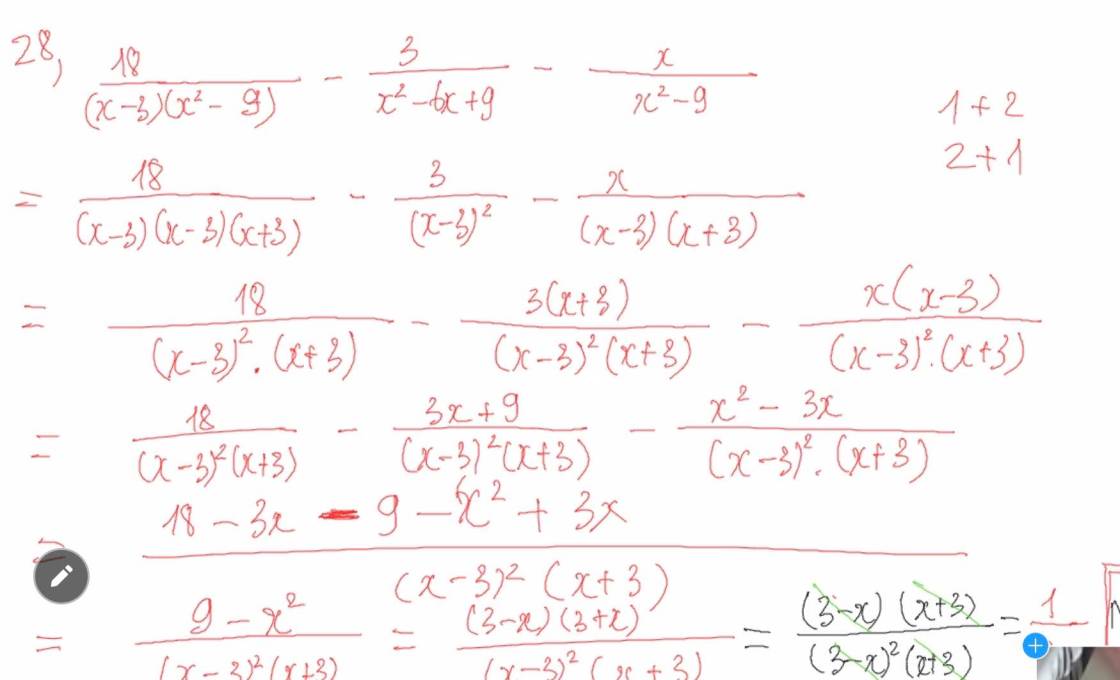
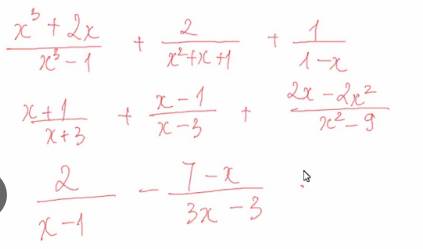

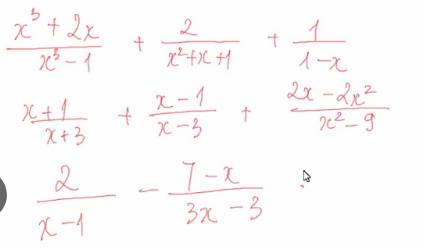
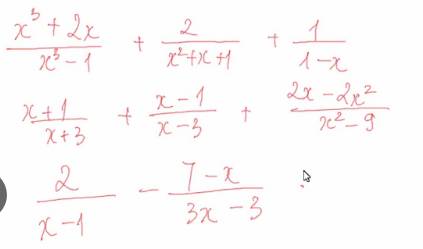


a) $KOH,Ba(OH)_2$
b)
$2Al(OH)_3 + 3H_2SO_4 \to Al_2(SO_4)_3 + 6H_2O$
$Fe(OH)_2 + H_2SO_4 \to FeSO_4 + 2H_2O$
$2Fe(OH)_3 + 3H_2SO_4 \to Fe_2(SO_4)_3 + 6H_2O$
$2KOH + H_2SO_4 \to K_2SO_4+ 2H_2O$
$Cu(OH)_2 + H_2SO_4 \to CuSO_4 + 2H_2O$
$Ba(OH)_2 + H_2SO_4 \to BaSO_4 + 2H_2O$
c)
$2KOH + SO_2 \to K_2SO_3 + H_2O$
$Ba(OH)_2 + SO_2 \to BaSO_3 + H_2O$
d)
$CuSO_4 + 2KOH \to Cu(OH)_2 + K_2SO_4$
$CuSO_4 + Ba(OH)_2 \to BaSO_4 + Cu(OH)_2$
e)
$2Al(OH)_3 \xrightarrow{t^o} Al_2O_3 + 3H_2O$
$Fe(OH)_2 \xrightarrow{t^o} FeO + H_2O$
$2Fe(OH)_3 \xrightarrow{t^o} Fe_2O_3 + 3H_2O$
$Cu(OH)_2 \xrightarrow{t^o} CuO + H_2O$
Bài 4:
\(a.\)
Chất làm quỳ tím hóa xanh : KOH , Ba(OH)2
\(b.\)
\(2Al\left(OH\right)_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2O\)
\(Fe\left(OH\right)_2+H_2SO_4\rightarrow FeSO_4+2H_2O\)
\(2Fe\left(OH\right)_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
\(KOH+H_2SO_4\rightarrow K_2SO_4+H_2O\)
\(Cu\left(OH\right)_2+H_2SO_4\rightarrow CuSO_4+2H_2O\)
\(Ba\left(OH\right)_2+H_2SO_4\rightarrow BaSO_4+2H_2O\)
\(c.\)
\(2KOH+SO_2\rightarrow K_2SO_3+H_2O\)
\(Ba\left(OH\right)_2+SO_2\rightarrow BaSO_3+H_2O\)
\(d.\)
\(2KOH+CuSO_4\rightarrow Cu\left(OH\right)_2+K_2SO_4\)
\(Ba\left(OH\right)_2+CuSO_4\rightarrow BaSO_4+Cu\left(OH\right)_2\)
\(e.\)
\(2Al\left(OH\right)_3\underrightarrow{^{^{t^0}}}Al_2O_3+3H_2O\)
\(2Fe\left(OH\right)_3\underrightarrow{^{^{t^0}}}Fe_2O_3+3H_2O\)
\(4Fe\left(OH\right)_2+O_2\underrightarrow{^{^{t^0}}}2Fe_2O_3+4H_2O\)
\(Cu\left(OH\right)_2\underrightarrow{^{^{t^0}}}CuO+H_2O\)