
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) nNaOH=20/40=0,5(mol)
nN2=1,12/22,4=0,05(mol)
nNH3= (0,6.1023)/(6.1023)=0,1(mol)
b) mAl2O3= 102.0,15= 15,3(g)
mSO2= nSO2 . M(SO2)= V(CO2,đktc)/22,4 . 64= 6,72/22,4. 64= 0,3. 64= 19,2(g)
mH2S= nH2S. M(H2S)= (0,6.1023)/(6.1023) . 34=0,1. 34 = 3,4(g)
c) V(CO2,đktc)=0,2.22.4=4,48(l)
nSO2=16/64=0,25(mol) -> V(SO2,đktc)=0,25.22,4=5,6(l)
nCH4=(2,1.1023)/(6.1023)=0,35(mol) -> V(CH4,đktc)=0,35.22,4=7,84(l)

\(3.1.\left(a\right)M_P=31\left(g/mol\right);\\ M_{Fe}=56\left(g/mol\right);\\ M_{H_2}=2\left(g/mol\right);\\ M_{O_2}=32\left(g/mol\right)\\ \left(b\right).M_{P_2O_5}=31.2+16.5=142\left(g/mol\right);\\ M_{Fe_3O_4}=56.3+16.4=232\left(g/mol\right);\\ M_{HCl}=1+35,5=36,5\left(g/mol\right);\\ M_{BaO}=137+16=153\left(g/mol\right)\\ c.M_{H_2SO_4}=2+32+16.4=98\left(g/mol\right);\\ M_{ZnCl_2}=65+35,5.2=136\left(g/mol\right);\\ M_{Al_2\left(SO_4\right)_3}=27.2+\left(32+16.4\right).3=342\left(g/mol\right);\\ M_{Ca\left(OH\right)_2}=40+17.2=74\left(g/mol\right)\)
\(3.2\left(a\right).n_{CH_4}=\dfrac{2,8}{22,4}=0,125\left(mol\right)\\ n_{O_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\\ \left(b\right).n_{CuO}=\dfrac{2}{80}=0,025\left(mol\right)\\ n_{Al_2\left(SO_4\right)_3}=\dfrac{3,42}{342}=0,01\left(mol\right)\)

Bài 5:
\(m_{Y}=m_{SO_2}+m_{CH_4}=\dfrac{3,36}{22,4}.64+\dfrac{13,44}{22,4}.16=19,2(g)\)
Bài 6:
\(V_{CO_2}=0,15.22,4=3,36(l)\\ V_{NO_2}=0,2.22,4=4,48(l)\\ V_{SO_2}=0,02.22,4=0,448(l)\\ V_{N_2}=0,03.22,4=0,672(l)\)

\(n_{O_2}=\dfrac{16}{32}=0,5\left(mol\right)=>V_{O_2}=0,5.22,4=11,2\left(l\right)\)
=> A
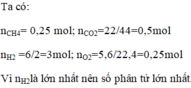
\(1,nCO_2=\dfrac{V}{22,4}=\dfrac{13.44}{22.4}=0.6\left(mol\right)\)
\(2,nSO_2=\dfrac{V}{22.4}=\dfrac{5.6}{22.4}=0.25\left(mol\right)\)
\(3,nO_2=\dfrac{m}{M}=\dfrac{16}{32}=0.5\left(mol\right)\)
\(4,nCH_4=\dfrac{m}{M}=\dfrac{32}{16}=2\left(mol\right)\)