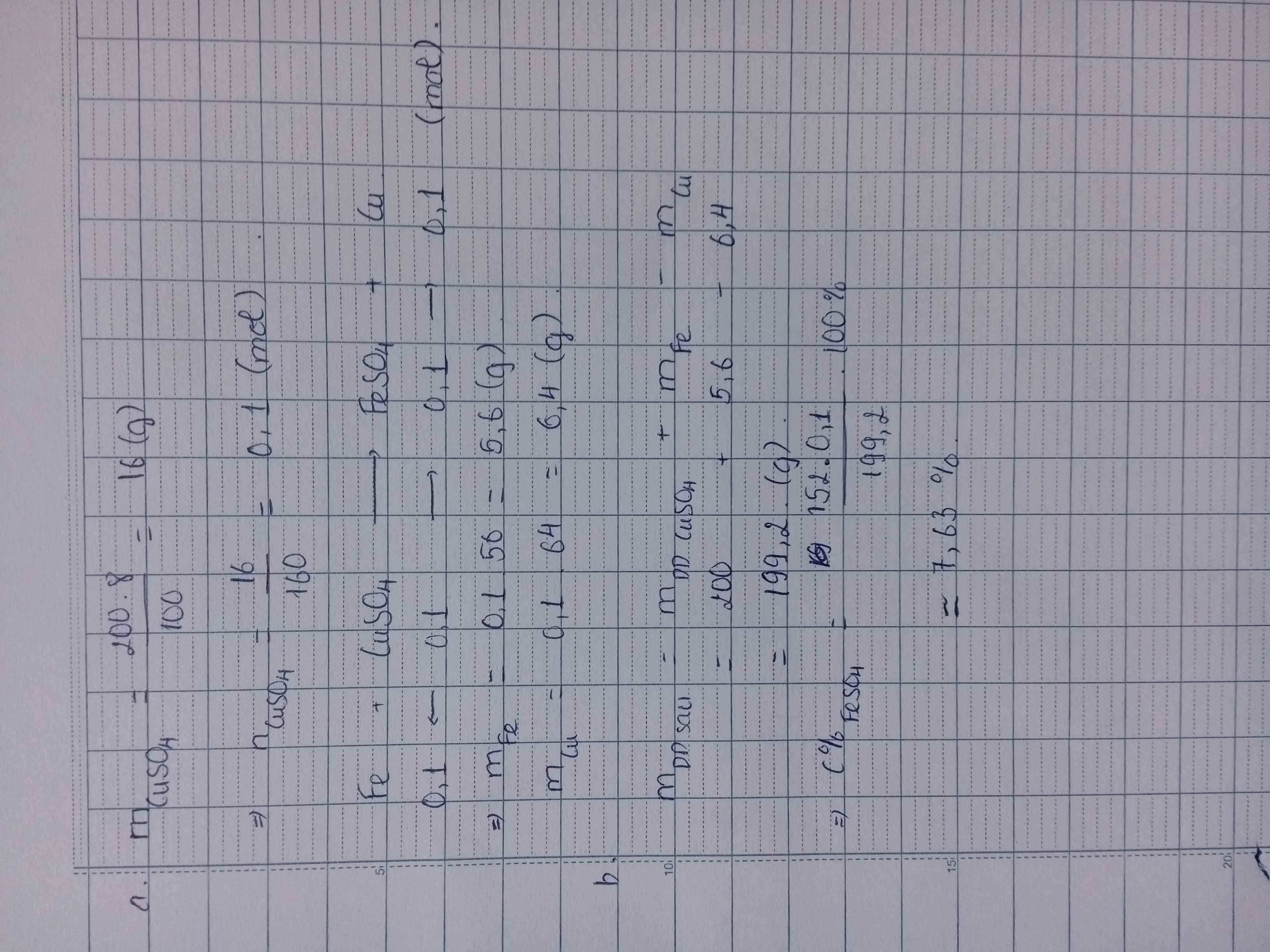Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
2x 3x x 3x (mol)
Ta có: \(m_{ddsaup/ứ}=m_{Al}+m_{ddH_2SO_4}-m_{H_2}=54x+200-6x\left(g\right)\)
Dung dịch muối có nồng độ 10%
\(\Rightarrow\dfrac{342x}{54x+200-6x}=0,1\) \(\Rightarrow x=\dfrac{50}{843}\left(mol\right)\)
\(\Rightarrow C\%_{H_2SO_4}=a\%=\dfrac{\dfrac{50}{243}\cdot98}{200}\cdot100\%\approx10,08\%\)

\(m_{ZnSO_4}=\dfrac{241,5.10}{100}=24,15\left(g\right)=>n_{ZnSO_4}=\dfrac{24,15}{161}=0,15\left(mol\right)\)
PTHH: 2Al + 3ZnSO4 --> Al2(SO4)3 + 3Zn
_____0,1<----0,15-------->0,05----->0,15
=> mAl = 0,1.27 = 2,7(g)
=> mZn = 0,15.65=9,75(g)
b) mdd sau pư = 2,7 + 241,5 - 9,75 = 234,45(g)
=> \(C\%\left(Al_2\left(SO_4\right)_3\right)=\dfrac{0,05.342}{234,45}.100\%=7,294\%\)

Ta có: \(m_{CuSO_4}=40.10\%=4\left(g\right)\Rightarrow n_{CuSO_4}=\dfrac{4}{160}=0,025\left(mol\right)\)
PT: \(Zn+CuSO_4\rightarrow ZnSO_4+Cu\)
Theo PT: \(n_{Zn}=n_{ZnSO_4}=n_{Cu}=n_{CuSO_4}=0,025\left(mol\right)\)
\(\Rightarrow m_{Zn}=0,025.65=1,625\left(g\right)\)
Ta có: m dd sau pư = 1,625 + 40 - 0,025.64 = 40,025 (g)
\(\Rightarrow C\%_{ZnSO_4}=\dfrac{0,025.161}{40,025}.100\%\approx10,056\%\)

\(n_{Al}=\dfrac{10,8}{27}=0,4(mol)\\ a,2Al+3H_2SO_4\to Al_2(SO_4)_3+3H_2\\ \Rightarrow n_{H_2}=n_{H_2SO_4}=0,6(mol);n_{Al_2(SO_4)_3}=0,2(mol)\\ b,V_{H_2}=0,6.22,4=13,44(l)\\ c,m_{dd_{H_2SO_4}}=\dfrac{0,6.98}{9,8\%}=600(g)\\ \Rightarrow C\%_{Al_2(SO_4)_3}=\dfrac{0,2.342}{10,8+600-0,6.2}.100\%=11,22\%\)

PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
Ta có: \(n_{H_2SO_4}=0,2\cdot1,5=0,3\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{Al}=0,2\left(mol\right)\\n_{Al_2\left(SO_4\right)_3}=0,1\left(mol\right)\\n_{H_2}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Al}=0,2\cdot27=5,4\left(g\right)\\V_{H_2}=0,3\cdot22,4=6,72\left(l\right)\\C_{M_{Al_2\left(SO_4\right)_3}}=\dfrac{0,1}{0,2}=0,5\left(M\right)\end{matrix}\right.\)

\(n_{CuSO_4}=\dfrac{200.8}{100.160}=0,1(mol)\\ PTHH:Fe+CuSO_4\to FeSO_4+Cu\\ a,n_{Cu}=n_{Fe}=n_{CuSO_4}=0,1(mol)\\ \Rightarrow m_{Cu}=0,1.64=6,4(g);m_{Fe}=0,1.56=5,6(g)\\ b,n_{FeSO_4}=0,1(mol)\\ \Rightarrow C\%_{FeSO_4}=\dfrac{0,1.152}{5,6+200-6,4}.100\%=7,63\%\)