
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


alo giúp mik Nhân dịp đi du lịch về, Mai mang đến lớp 51 móc chìa khóa để tặng cho cácbạn. Sau khi chia hết cho các bạn (tính cả mình) thì Mai còn thừa3chiếc. Hỏi lớpMai có bao nhiêu bạn, biết số học sinh nhiều hơn 20 và ít hơn 30 bạn.

X: Fe3O4
Y: FeCl2
Z: FeCl3
T: Fe(OH)2
U: Fe(OH)3
A: NaCl (hoặc H2O)
B: H2O (hoặc NaCl)
D: H2 (hoặc Cl2)
E: Cl2 (hoặc H2)
F: NaOH
G: HCl
PTHH:
a) NaCl + H2O -dpmn----> 1/2 H2 + 1/2 Cl2 + NaOH
H2 + Cl2 -to-> 2 HCl
HCl + NaOH -> NaCl + H2O
b) 3 Fe +2 O2 -to->Fe3O4
Fe3O4 + 8 HCl -> FeCl2 +2 FeCl3 + H2O
FeCl2 + 2 NaOH -> Fe(OH)2 + 2 NaCl
FeCl3 +3 NaOH -> Fe(OH)3 + 3NaCl
Chúc em học tốt!

$2Fe + 6H_2SO_4 \to Fe_2(SO_4)_3 + 3SO_2 + 6H_2O(1)$
$Fe_2(SO_4)_3 + Fe\ to 3FeSO_4(2)$
Gọi $n_{Fe_2(SO_4)_3} = a(mol) ; n_{FeSO_4} = b(mol)$
Ta có : $400a + 152b = 62,8(1)$
$n_{SO_2} = 0,15(mol)$
$n_{Fe_2(SO_4)_3(1)} = \dfrac{1}{3}n_{SO_2} = 0,05(mol)$
$n_{Fe_2(SO_4)_3(2)} = \dfrac{1}{3}n_{FeSO_4} = \dfrac{b}{3}$
Suy ra:
$0,05 - \dfrac{b}{3} = a(2)$
Từ (1)(2) suy ra $a = \dfrac{45}{112} ; b = -1,055<0$
=> Sai đề

Bài 2) Ở 90 độ C:
- 100 gam nước hoà tan 50 gam KCl để tạo 150 gam dung dịch bão hoà ở nhiệt độ này
a) C% của dung dịch bão hoà tại 90 độ C là:
(Khối lượng chất tan/Khối lượng dung dịch) . 100%
<=> (50:150).100% = 33,33%
b) Ở 0 độ C:
Gọi m là khối lượng chất tan KCl ở 0o C => Khối lượng dung dịch tại nhiệt độ này là: 100+m
Theo đề bài ra ta có: m/100+m = 25,93%
=> m = 35 gam
Vậy ở 0 độ C độ tan của KCl trong nước là 35 gam
c) Ở 90 độ C:
100 gam nước hoà tan 50 gam KCl tạo 150 gam dd
=> 600 gam dung dịch tạo 200 gam KCl và 400 gam nước
- Ở 0 độ C:
100 gam nước hoà tan 35 gam KCl tạo 135 gam dd
=> 400 gam nước hoà tan được 140 gam KCl tạo 400 + 140 = 540 gam dung dịch
Vậy khi làm lạnh 600 gam dung dịch KCl từ 90 độ xuống 0 độ thì khối lượng dung dịch thu được là 540 gam


A là dung dịch H2SO4
B: Na2CO3
C: H2SO4 đặc
D: Xút (NaOH)
Khi cho DD H2SO4 tác dụng với Na2CO3 giải phóng khí SO2 mang theo hơi nước.
Bình C để giữ hơi nước lại trong bình (H2SO4 đặc háu nước) SO2 không tác dụng tiếp tục được dẫn qua bình đựng.
Để tránh SO2 thoát ra bên ngoài gây ô nhiễm môi trường và 1 số bệnh cho con người nên Xút được đặt ở miệng bình để tạo muối.

12.
Na2CO3+H2SO4->Na2SO4+H2O+CO2
............. 0,5 ............. ......... 0,5
CO2+2KOH->K2CO3+H2O
x 2x x
CO2+KOH->KHCO3
y y y
mKOH=98.40/100=39,2g
nKOH=39,2/56=0,7mol
Có:
2x+y=0,7
138x+100y=57,6
=>x=0,2mol; y=0,3mol
mK2CO3=138.0,2=27,6g
mKHCO3=57,6-27,6=30g
b.
nCO2=x+y=0,2+0,3=0,5mol
CMddH2SO4=0,5/0,2=2,5M
8. Hoàn thành sơ đồ chuyển hóa sau:
Mg \(\underrightarrow{\left(1\right)}\) MgO \(\underrightarrow{\left(2\right)}\) MgCl2 \(\underrightarrow{\left(3\right)}\) Mg(OH)2 \(\underrightarrow{\left(4\right)}\) MgO \(\underrightarrow{\left(5\right)}\) MgSO4 \(\underrightarrow{\left(6\right)}\) MgCO3 \(\underrightarrow{\left(7\right)}\) MgO
\(\left(1\right)2Mg+O_2\underrightarrow{t^o}2MgO\)
\(\left(2\right)MgO+2HCl\rightarrow MgCl_2+H_2O\)
\(\left(3\right)MgCl_2+2NaOH\rightarrow Mg\left(OH\right)_2\downarrow+2NaCl\)
\(\left(4\right)Mg\left(OH\right)_2\underrightarrow{t^o}MgO+H_2O\)
\(\left(5\right)MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
\(\left(6\right)MgSO_4+Na_2CO_3\rightarrow MgCO_3+Na_2SO_4\)
\(\left(7\right)MgCO_3\underrightarrow{t^o}MgO+CO_2\uparrow\)


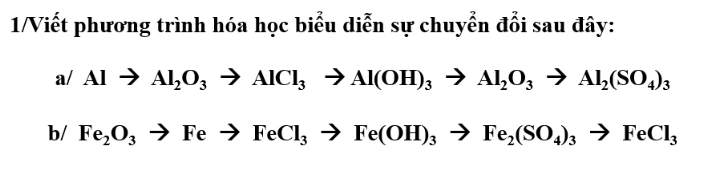


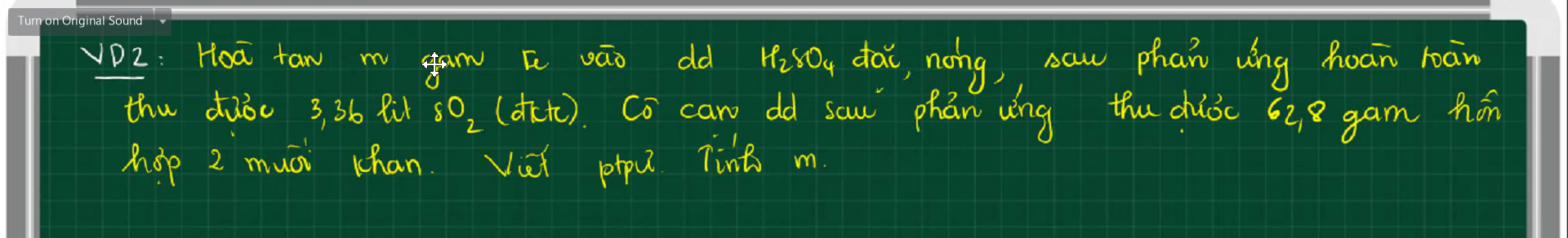





 Giúp hộ mình nha !!!
Giúp hộ mình nha !!!





 Giúp mình nha !!!
Giúp mình nha !!!