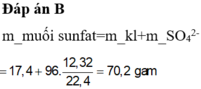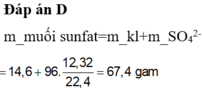Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đáp án D.
nSO2 = 0,55 => ne = 0,55.2 = 1,1 (mol)
mmuối = mKL + Mgốc axit. ne/2
= 14,6 + 96. 1,1/2 = 67,4 g

a, \(Fe+H_2SO_{4\text{loãng}}\rightarrow FeSO_4+H_2\)
\(n_{Fe}=n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
\(Fe+H_2SO_{4\text{đặc}}\rightarrow Fe_2\left(SO_4\right)_3+SO_2+H_2O\)
\(Cu+H_2SO_{4\text{đặc}}\rightarrow CuSO_4+SO_2+H_2O\)
Bảo toàn e:
\(2n_{Cu}+3n_{Fe}=2n_{SO_2}\)
\(\Leftrightarrow n_{Cu}=\dfrac{2n_{SO_2}-3n_{Fe}}{2}=0,25\left(mol\right)\)
\(\Rightarrow x=m_{Cu}+m_{Fe}=0,25.64+0,5.56=44\left(g\right)\)
a) Đặt \(\left\{{}\begin{matrix}n_{Cu}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\end{matrix}\right.\)
Ta có: \(\left\{{}\begin{matrix}n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)=b=n_{Fe}\\n_{SO_2}=\dfrac{22,4}{22,4}=1\left(mol\right)\end{matrix}\right.\)
Bảo toàn electron: \(2a+3b=2\) \(\Rightarrow2a+3\cdot0,5=2\) \(\Rightarrow a=n_{Cu}=0,25\left(mol\right)\)
\(\Rightarrow x=m_{Cu}+m_{Fe}=0,25\cdot64+0,5\cdot56=44\left(g\right)\)
b) Ta có: \(n_{H_2SO_4\left(p/ư\right)}=\dfrac{1}{2}n_{e\left(traođổi\right)}+n_{SO_2}=\dfrac{1}{2}\cdot2+1=2\left(mol\right)\)
\(\Rightarrow\Sigma n_{H_2SO_4\left(đặc\right)}=2\cdot110\%=2,2\left(mol\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{2,2\cdot98}{98\%}=220\left(g\right)\) \(\Rightarrow V_{H_2SO_4}=\dfrac{220}{1,84}\approx119,57\left(ml\right)\)
c) Ta có: \(\left\{{}\begin{matrix}n_{SO_2}=1\left(mol\right)\\n_{Ba\left(OH\right)_2}=0,4\cdot1,5=0,6\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Tạo 2 muối
PTHH: \(2SO_2+Ba\left(OH\right)_2\rightarrow Ba\left(HSO_3\right)_2\)
2x x x (mol)
\(SO_2+Ba\left(OH\right)_2\rightarrow BaSO_3\downarrow+H_2O\)
y y (mol)
Ta lập được hệ phương trình: \(\left\{{}\begin{matrix}x+y=0,6\\2x+y=1\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=n_{Ba\left(HSO_3\right)_2}=0,4\left(mol\right)\\y=0,2\end{matrix}\right.\)
\(\Rightarrow C_{M_{Ba\left(HSO_3\right)_2}}=\dfrac{0,4}{0,4}=1\left(M\right)\)

\(n_{Cu}=a\left(mol\right),n_{Fe}=b\left(mol\right)\)
\(m_X=64a+56b=16.2\left(g\right)\left(1\right)\)
\(n_{SO_2}=\dfrac{8.96}{22.4}=0.4\left(mol\right)\)
Bảo toàn e :
\(2a+3b=0.4\cdot2=0.8\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.0475,b=0.235\)
\(\%Cu=\dfrac{0.0475\cdot64}{16.2}\cdot100\%=18.76\%\)
\(\%Fe=81.24\%\)
\(b.\)
\(\dfrac{a}{b}=\dfrac{0.0475}{0.235}=\dfrac{19}{94}\)
\(\Rightarrow n_{Cu}=19x\left(mol\right),n_{Fe}=94x\left(mol\right)\)
\(m_X=19x\cdot64+94x\cdot56=22\left(g\right)\)
\(\Rightarrow x=\dfrac{11}{3240}\)
\(n_{H_2}=n_{Fe}=\dfrac{11}{3240}\cdot94=\dfrac{517}{1620}\left(mol\right)\)
\(V_{H_2}=7.15\left(l\right)\)