Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đáp án B
Cho Al và Ag phản ứng với H 2 S O 4 loãng, dư chỉ có Al phản ứng.
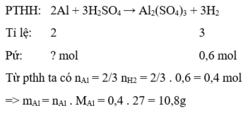
% m A l = 10,8 12 .100 % = 90 % .
% m A g = 100 % - 90 % = 10 %

m(rắn)=mAg=3(g); nH2=6,72/22,4=0,3(mol)
2 Al +3 H2SO4 -> Al2(SO4)3 + 3 H2
nAl=2/3. 0,3=0,2(mol) => mAl=0,2.27=5,4(g)
=> \(\%mAl=\dfrac{5,4}{5,4+3}.100\approx64,3\%\)
=> CHỌN B
\(n_{H_2}=0,3\left(mol\right)\)
\(2Al+3H_2SO_{4\left(l\right)}\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,2<---------------------------------------0,3
\(m_{Al}=0,2.27=5,4\left(g\right)\)
\(m_{r_{bđ}}=5,4+3=8,4\left(g\right)\)
\(\%m_{Al}=\dfrac{5,4.100\%}{8,4}\approx64,3\%\)
=>B

Câu 2:
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ n_{H_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\\ n_{Al}=\dfrac{2.0,6}{3}=0,4\left(mol\right)\\ \%m_{Al}=\dfrac{0,4.27}{12}.100\%=90\%\Rightarrow\%m_{Ag}=100\%-90\%=10\%\)
Câu 3:
\(n_{H_2}=\dfrac{0,6}{2}=0,3\left(mol\right)\\ 2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\\ n_{Al}=\dfrac{2}{3}.0,3=0,2\left(mol\right)\\ n_{Al_2O_3}=\dfrac{25,8-0,2.27}{102}=0,2\left(mol\right)\\ n_{AlCl_3}=n_{Al}+2n_{Al_2O_3}=0,2+2.0,2=0,6\left(mol\right)\\ m_{AlCl_3}=133,5.0,6=80,1\left(g\right)\)

PT: \(Mg+H_2SO_4\rightarrow MgSO_4+H_2\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
a, Giả sử: \(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\n_{Al}=y\left(mol\right)\end{matrix}\right.\)
⇒ 24x + 27y = 12,6 (1)
Ta có: \(n_{H_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Mg}+\dfrac{3}{2}n_{Al}=x+\dfrac{3}{2}y\left(mol\right)\)
\(\Rightarrow x+\dfrac{3}{2}y=0,6\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,3\left(mol\right)\\y=0,2\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{MG}=\dfrac{0,3.24}{12,6}.100\%\approx57,1\%\\\%m_{Al}\approx42,9\%\end{matrix}\right.\)
b, Theo PT: \(\left\{{}\begin{matrix}n_{H_2SO_4}=n_{H_2}=0,6\left(mol\right)\\n_{MgSO_4}=n_{Mg}=0,3\left(mol\right)\\n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{H_2SO_4}=0,6.98=58,8\left(g\right)\Rightarrow m_{ddH_2SO_4}=\dfrac{58,8}{14,7\%}=400\left(g\right)\)
Ta có: m dd sau pư = 12,6 + 400 - 0,6.2 = 411,4 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{MgSO_4}=\dfrac{0,3.120}{411,4}.100\%\approx8,75\%\\C\%_{Al_2\left(SO_4\right)_3}=\dfrac{0,1.342}{411,4}.100\%\approx8,31\%\end{matrix}\right.\)
Bạn tham khảo nhé!

Theo bài ra, ta có: \(m_{Ag}=5,6\left(g\right)\)
PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
a) Ta có: \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\) \(\Rightarrow n_{Al}=\dfrac{1}{15}\left(mol\right)\) \(\Rightarrow m_{Al}=\dfrac{1}{15}\cdot27=1,8\left(g\right)\)
\(\Rightarrow\%m_{Al}=\dfrac{1,8}{1,8+5,6}\cdot100\%\approx24,32\%\) \(\Rightarrow\%m_{Ag}=75,68\%\)
b) Theo PTHH: \(n_{H_2SO_4}=n_{H_2}=0,1mol\) \(\Rightarrow C_{M_{H_2SO_4}}=\dfrac{0,1}{0,1}=1\left(M\right)\)
c) PTHH: \(H_2SO_4+Ba\left(OH\right)_2\rightarrow BaSO_4\downarrow+2H_2O\)
Theo PTHH: \(n_{Ba\left(OH\right)_2}=n_{H_2SO_4}=0,1mol\)
\(\Rightarrow V_{ddBa\left(OH\right)_2}=\dfrac{0,1}{0,2}=0,5\left(l\right)=500\left(ml\right)\)

\(n_{H_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
_____0,4<-----------------------------------0,6
=> \(\%Al=\dfrac{0,4.27}{12}.100\%=90\%\)
%Ag = 100% - 90% = 10%