Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Cau 1 :
\(n_{H2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,1 0,1
a) Chat trong dung dich A thu duoc la : sat (II) clorua
Chat ran B la : dong
Chat khi C la : khi hidro
b) \(n_{Fe}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
\(m_{Fe}=0,1.56=5,6\left(g\right)\)
\(m_{Cu}=10-5,6=4,4\left(g\right)\)
0/0Fe = \(\dfrac{5,6.100}{10}=56\)0/0
0/0Cu = \(\dfrac{4,4.100}{10}=44\)0/0
c) Co : \(m_{Cu}=4,4\left(g\right)\)
\(n_{Cu}=\dfrac{4,4}{64}=0,06875\left(mol\right)\)
Pt : \(Cu+2H_2SO_{4dac}\underrightarrow{t^o}CuSO_4+SO_2+2H_2O|\)
1 2 1 1 2
0,06875 0,06875
\(n_{SO2}=\dfrac{0,06875.1}{1}=0,06875\left(mol\right)\)
\(V_{SO2\left(dktc\right)}=1,54\left(l\right)\)
Chuc ban hoc tot
Minh xin loi ban nhe , ban bo sung vao cho :
\(V_{SO2\left(dktc\right)}=0,06875.22,4=1,54\left(l\right)\)

PTHH:
\(CaCO_3+2HCl\rightarrow CaCl_2+H_2O+CO_2\uparrow\)
0,15 0,15
\(CaO+2HCl\rightarrow CaCl_2+H_2O\)
Ta có: \(n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(\Rightarrow m_{CaCO_3}=0,15\cdot100=15\left(g\right)\)
\(\Rightarrow m_{CaO}=20,6-15=5,6\left(g\right)\)
\(\Rightarrow\%m_{CaCO_3}=\dfrac{15\cdot100}{20,6}\approx73\%\)
\(\Rightarrow\%m_{CaO}=100\%-73\%=27\%\)

\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: Fe + H2SO4 → FeSO4 + H2
Mol: 0,4 0,4
\(m_{Fe}=0,4.56=22,4\left(g\right)\)
\(m_{hh}=22,4+5=27,4\left(g\right)\)
\(\%m_{Fe}=\dfrac{22,4.100\%}{27,4}=81,75\%;\%m_{Cu}=100-81,75=18,25\%\)

a. PTHH:
Cu + HCl ---x--->
Fe + 2HCl ---> FeCl2 + H2
Vậy chất rắn A là Cu.
b. Ta có: \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{Fe}=n_{H_2}=0,3\left(mol\right)\)
=> \(m_{Fe}=0,3.56=16,8\left(g\right)\)
=> \(\%_{m_{Fe}}=\dfrac{16,8}{30}.100\%=56\%\)
\(\%_{m_{Cu}}=100\%-56\%=44\%\%\)
c.
Theo PT: \(n_{FeCl_2}=n_{Fe}=0,3\left(mol\right)\)
=> \(m_{FeCl_2}=0,3.127=38,1\left(g\right)\)
d.
Ta có: \(m_{dd_{FeCl_2}}=100+16,8=116,8\left(g\right)\)
=> \(C_{\%_{FeCl_2}}=\dfrac{38,1}{116,8}.100\%=32,62\%\)
ta có Cu ko phản ứng với HCl
-> V khí là do Fe phản ứng hết tạo ra
Fe + 2HCl -> FeCl2 + H2
0,3 .............................0,3
n H2 = 6,72 : 22,4=0,3 mol
m Fe = 0,3.56 =16,8 g
% Fe = 16,8 : 30 .100 = 56 %
% Cu = 100% - 56% = 44%

a) \(2Al+6HCl\rightarrow2AlCl_3+3H_2\left(1\right)\\ Fe+2HCl\rightarrow FeCl_2+H_2\left(2\right)\\ 2Al+2NaOH+2H_2O\rightarrow2NaAlO_2+3H_2\)
Cho hỗn hợp tác dụng với NaOH, chất rắn không tan là Fe
=> mFe= 1,12 (g) \(\Rightarrow n_{Fe}=0,02\left(mol\right)\)
Ta có: \(n_{H_2\left(2\right)}=n_{Fe}=0,02\left(mol\right)\)
=> \(n_{H_2\left(1\right)}=\Sigma n_{H_2}-n_{H_2\left(2\right)}=0,065-0,02=0,045\left(mol\right)\)
\(\Rightarrow n_{Al}=\dfrac{2}{3}n_{H_2\left(1\right)}=0,03\left(mol\right)\)
\(\Rightarrow m_{Al}=0,03.27=0,81\left(g\right)\)
\(\Rightarrow\%m_{Al}=41,97\%,\%m_{Fe}=58,03\%\)
b) \(m_{FeCl_2}=0,02.127=2,54\left(g\right)\\ m_{AlCl_3}=0,03.133,5=4,005\left(g\right)\)

a)
$Fe + 2HCl \to FeCl_2 + H_2$
$FeO +2 HCl \to FeCl_2 + H_2O$
b)
Theo PTHH :
$n_{Fe} = n_{H_2} = \dfrac{4,48}{22,4} = 0,2(mol)$
$\%m_{Fe} = \dfrac{0,2.56}{20}.100\% = 56\%$
$\%m_{FeO} = 100\% - 56\% = 44\%$
c) $n_{FeO} = \dfrac{11}{90}(mol)$
$n_{HCl} = 2n_{Fe} + 2n_{FeO} = \dfrac{29}{45}(mol)$
$m_{dd\ HCl} = \dfrac{ \dfrac{29}{45}.36,5}{7,3\%} = 322,22(gam)$
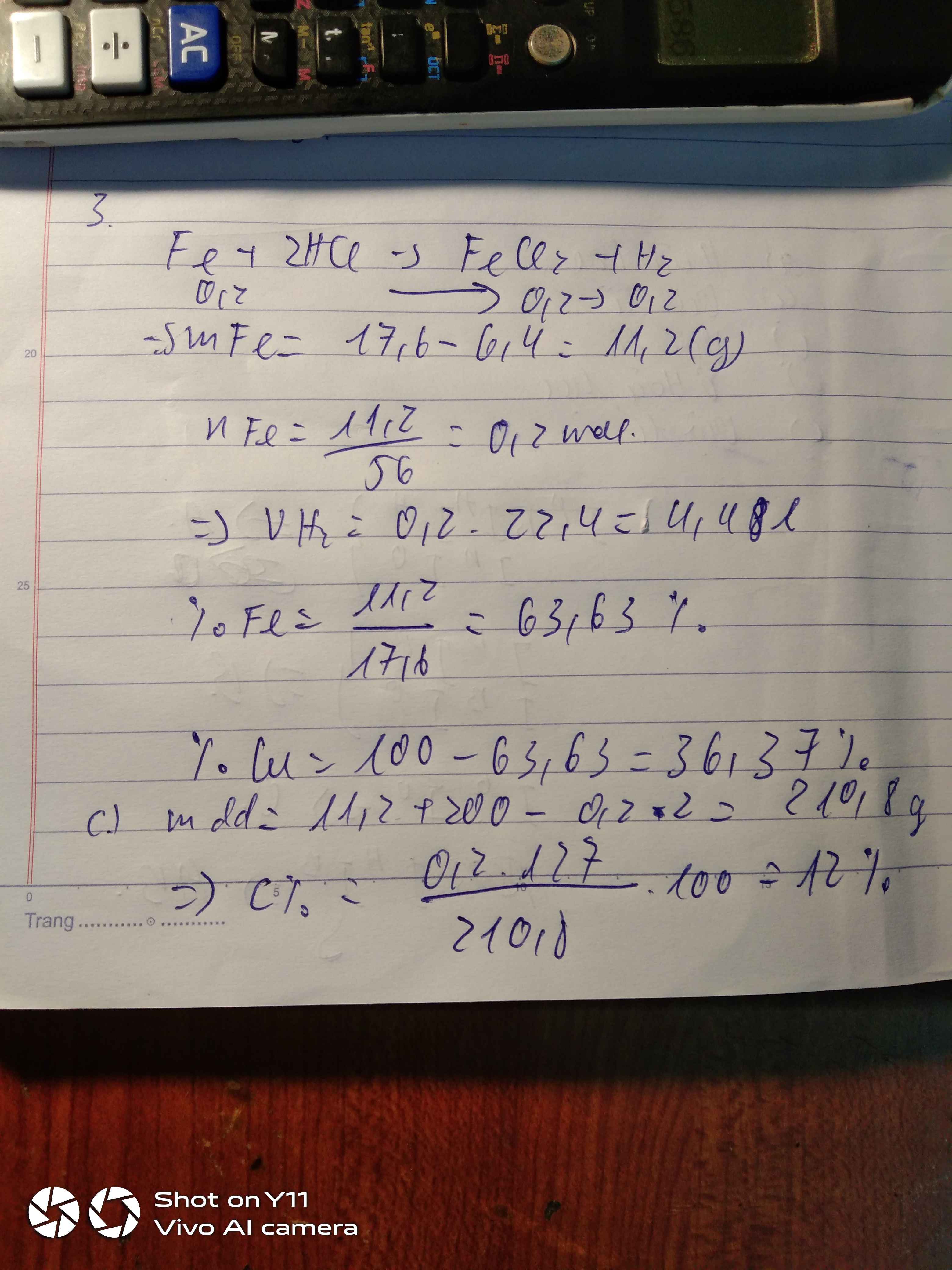
a) PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
b) Theo bài ra, ta có: \(m_{Cu}=3,2\left(g\right)\)
\(\Rightarrow\%m_{Cu}=\dfrac{3,2}{12}\cdot100\%\approx26,67\%\) \(\Rightarrow\%m_{Fe}=73,33\%\)
c) Ta có: \(n_{Fe}=\dfrac{12-3,2}{56}=\dfrac{11}{70}\left(mol\right)=m_{FeCl_2}\)
\(\Rightarrow m_{FeCl_2}=\dfrac{11}{70}\cdot127\approx19,96\left(g\right)\)
a) PTHH: Fe+2HCl→FeCl2+H2↑
b) Theo bài ra, ta có: mCu=3,2(g)
⇒%mCu=3,212⋅100%≈26,67% ⇒%mFe=73,33%
c) Ta có: nFe=12−3,256=1170(mol)=mFeCl2
⇒mFeCl2=1170⋅127≈19,96(g)