Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a)2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ b)n_{H_2}=\dfrac{5,6}{22,4}=0,25mol\\ n_{Al}=a;n_{Fe}=b\\ \left\{{}\begin{matrix}3a+b=0,25\\27a+56b=8,3\end{matrix}\right.\\ a=\dfrac{19}{470};b=\dfrac{121}{940}\\ \%m_{Al}=\dfrac{\dfrac{19}{470}\cdot27}{8,3}\cdot100=13,15\%\\ \%m_{Fe}=100-13,15=86,85\%\\ c)n_{HCl}=3\cdot\dfrac{19}{470}+2\cdot\dfrac{121}{940}=\dfrac{89}{235}mol\\ m_{ddHCl=}=\dfrac{\dfrac{89}{235}\cdot36,5}{7,3}\cdot100=189g\\ d)n_{AlCl_3}=n_{Al}=\dfrac{19}{470}mol\\ n_{Fe}=n_{FeCl_2}=\dfrac{121}{940}mol\)
\(m_{dd}=8,3+189-0,25.2=196,8g\\ C_{\%AlCl_3}=\dfrac{\dfrac{19}{470}\cdot133,8}{196,8}\cdot100=2,8\%\\ C_{\%FeCl_2}=\dfrac{\dfrac{121}{940}127}{196,8}\cdot100=8,3\%\)

a)
$Fe + 2HCl \to FeCl_2 + H_2$
$FeO +2 HCl \to FeCl_2 + H_2O$
b)
Theo PTHH :
$n_{Fe} = n_{H_2} = \dfrac{4,48}{22,4} = 0,2(mol)$
$\%m_{Fe} = \dfrac{0,2.56}{20}.100\% = 56\%$
$\%m_{FeO} = 100\% - 56\% = 44\%$
c) $n_{FeO} = \dfrac{11}{90}(mol)$
$n_{HCl} = 2n_{Fe} + 2n_{FeO} = \dfrac{29}{45}(mol)$
$m_{dd\ HCl} = \dfrac{ \dfrac{29}{45}.36,5}{7,3\%} = 322,22(gam)$

n Na2CO3 = 42,4 / 106 =0,4(mol)
m HCl = (91,25 .20 %) / 100%= 18,25 (g) ---> n HCl= 18,25 / 36,5 =0,5 (mol)
Na2CO3 + 2HCl ---> 2NaCl + CO2 +H2O
Vì 0,4/1 > 0,5/2 nên sau phản ứng Na2CO3 dư , HCl hết
theo pthh ta có n CO2 = 1/2 n HCl = 1/2 . 0,5 =0,25(mol)
----> VCO2 = 0,25 . 22,4 = 5,6 (l)
theo pthh ta có n NaCl = n HCl = 0,5 (mol) ---> m NaCl = 0,5 . 58,5 =29,25 (g)
theo pthh ta có n NaCO3( pứ )= 1/2 n HCl = 1/2. 0,5 = 0,25 (mol)
---> n NaCO3(dư) = 0,4 - 0,25 = 0,15 (mol)
----> m Na2CO3 (dư) = 0,15 . 106 = 15,9 (g)
mddspứ=m Na2CO3 + m HCl - m CO2= 42,4 + 91,25 - 0,25. 44
=122,65 (g)
---> C% NaCl = ( 29,25 / 122,65 ). 100= 23,84%
----> C% Na2CO3 (dư) = ( 15,9 / 122,65 ). 100=13%
nNa2CO3=0,4mol
mHCl=18,25g=>nHCl=0,5mol
PTHH: Na2CO3+2HCl=>2NaCl+CO2+H2O
0,4: 0,5 => n Na2CO3 dư theo nHCl
p/ư; 0,25<---0,25---->0,5----->0,25--->0,25
V CO2=0,25.22,4=5,6 lít
m NaCl=0,5.58,5=29,25g
theo định luật bảo toàn khối lượng :
mdd NaCl=42,4+91,25-0,25.44-0,25.18=118,15g
=> C% naCl=29,25:118,15.100=24,76%

a) PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
b) Ta có: \(\left\{{}\begin{matrix}n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\\n_{HCl}=\dfrac{200\cdot10\%}{36,5}=\dfrac{40}{73}\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,2}{1}< \dfrac{\dfrac{40}{73}}{2}\) \(\Rightarrow\) HCl còn dư, Fe phản ứng hết
\(\Rightarrow n_{H_2}=0,2mol\) \(\Rightarrow V_{H_2}=0,2\cdot22,4=4,48\left(l\right)\)
c) PTHH: \(HCl+NaOH\rightarrow NaCl+H_2O\)
Ta có: \(n_{HCl\left(dư\right)}=\dfrac{54}{365}\left(mol\right)=n_{NaOH}\)
\(\Rightarrow V_{NaOH}=\dfrac{\dfrac{54}{365}}{0,5}\approx0,3\left(l\right)=300\left(ml\right)\)

\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
a) PTHH : \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
Theo Pt : \(n_{Fe}=n_{H2SO4}=n_{FeSO4}=n_{H2}=0,2\left(mol\right)\)
b) \(V_{H2\left(dktc\right)}=0,2.22,4=4,48\left(l\right)\)
c) \(C_{MddH2SO4}=\dfrac{0,2}{0,2}=1\left(M\right)\)
d) \(m_{muối}=m_{FeSO4}=0,2.152=30,4\left(g\right)\)

\(a,n_{Fe}=\dfrac{11,2}{56}=0,2(mol)\\ PTHH:Fe+2HCl\to FeCl_2+H_2\\ \Rightarrow n_{HCl}=0,4(mol)\\ \Rightarrow m_{dd_{HCl}}=\dfrac{0,4.36,5}{14,6\%}=100(g)\\ b,n_{H_2}=0,2(mol)\\ \Rightarrow V_{H_2}=0,2.22,4=4,48(l)\\ c,n_{FeCl_2}=0,2(mol)\\ \Rightarrow C\%_{FeCl_2}=\dfrac{0,2.127}{11,2+100-0,2.2}.100\%\approx 22,93\%\)

a) \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
Gọi \(\left\{{}\begin{matrix}n_{Al}:x\left(mol\right)\\n_{Fe}:y\left(mol\right)\end{matrix}\right.\)
Ta có : \(\left\{{}\begin{matrix}27x+56y=11\\1,5x+y=0,4\end{matrix}\right.\)
=> x=0,2 ; y=0,1
\(\%m_{Al}=\dfrac{0,2.27}{11}.100==49,09\%\)
\(\%m_{Fe}=50,91\%\)
b) \(\Sigma n_{HCl}=3x+2y=0,8\left(mol\right)\)
=> \(V_{HCl}=\dfrac{0,8}{2}=0,4\left(lít\right)\)
c) \(CM_{AlCl_3}=\dfrac{0,2}{0,4}=0,5M\)
\(CM_{FeCl_2}=\dfrac{0,1}{0,4}=0,25M\)
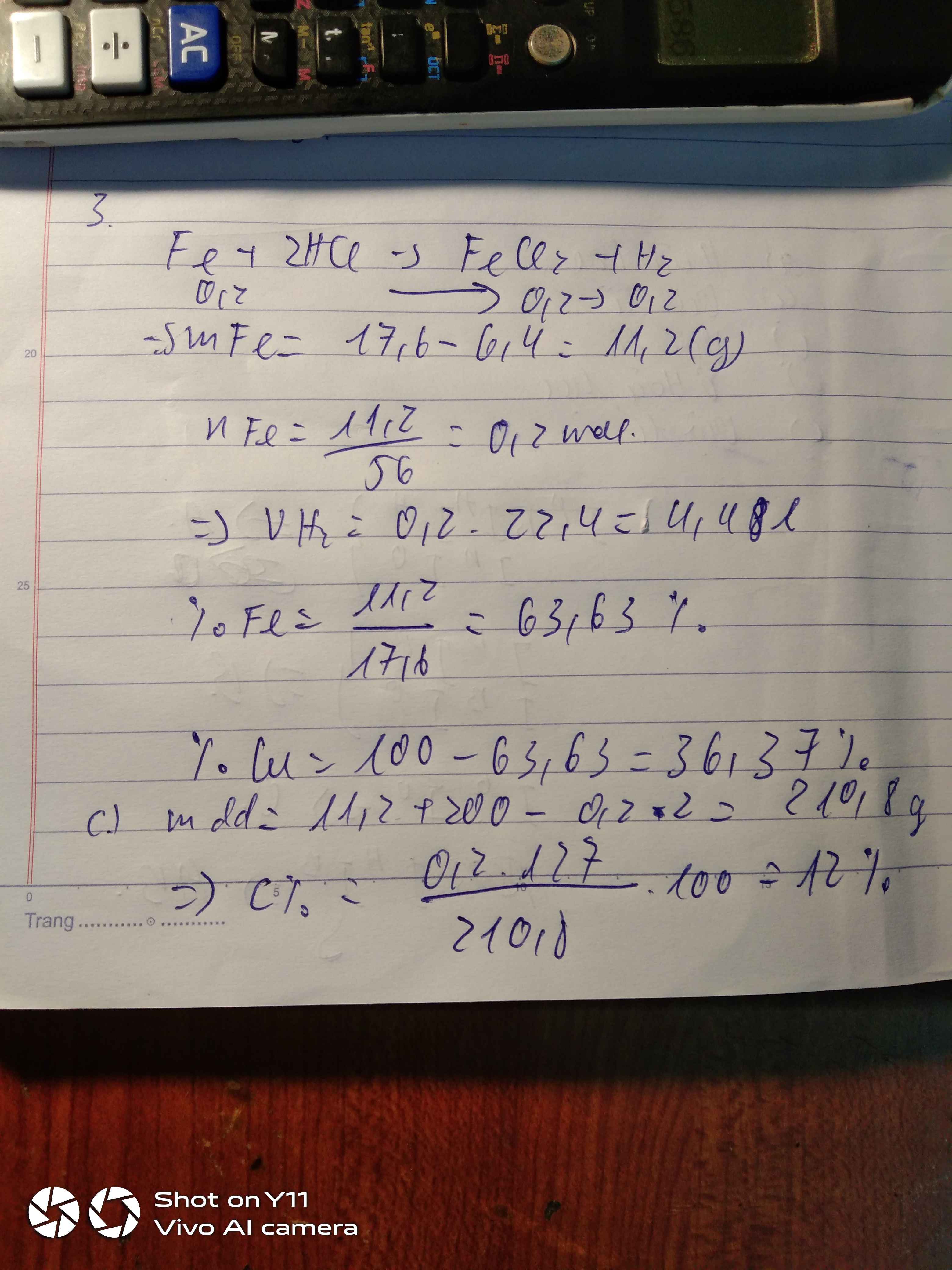
a) $Fe + 2HCl \to FeCl_2 + H_2$
b) $n_{Fe} = \dfrac{11,2}{56} = 0,2(mol)$
$n_{HCl} = \dfrac{200.7,3\%}{36,5} = 0,4(mol)$
$Fe + 2HCl \to FeCl_2 + H_2$
Ta thấy : $n_{Fe} : 1 = n_{HCl} : 2$ nên phản ứng vừa đủ
$n_{H_2} = n_{Fe} = 0,2(mol)$
$V_{H_2} = 0,2.22,4 = 4,48(lít)$
c) $m_{dd\ sau\ pư} = 11,2 + 200 - 0,2.2 = 210,8(gam)$
$C\%_{FeCl_2} = \dfrac{0,2.127}{210,8}.100\% = 12,05\%$
Em cảm ơn anh ạ