
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



a) 2KMnO4 +16HCl --> 2KCl + 2MnCl2 + 5Cl2 + 8H2O
Chất oxh: KMnO4; chất khử: HCl
| Mn+7 +5e->Mn+2 | x2 |
| 2Cl- -2e--> Cl20 | x5 |
b) 8Al + 30HNO3 --> 8Al(NO3)3 + 3N2O + 15H2O
| Al0 -3e --> Al+3 | x8 |
| 2N+5 +8e--> N2+1 | x3 |
31:
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
PTHH: Fe + H2SO4 --> FeSO4 + H2
_____0,1----------------->0,1
10FeSO4 + 2KMnO4 + 8H2SO4 --> K2SO4 + 2MnSO4 + 5Fe2(SO4)3 + 8H2O
=> nKMnO4 = 0,02 (mol)
=> \(V=\dfrac{0,02}{0,5}=0,04\left(l\right)=40\left(ml\right)\)



Câu 16:
PTHH: \(Cl_2+2NaOH\rightarrow NaCl+NaClO+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{Cl_2}=\dfrac{33,6}{22,4}=1,5\left(mol\right)\\n_{NaOH}=\dfrac{600\cdot20\%}{40}=3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) 2 chất p/ứ hết
Mặt khác: \(m_{Cl_2}=1,5\cdot71=106,5\left(g\right)\)
\(\Rightarrow m_{nướcjaven}=m_{Cl_2}+m_{ddNaOH}=706,5\left(g\right)\)

a.
\(n_S=\dfrac{16}{32}=0,5mol\)
Gọi \(\left\{{}\begin{matrix}n_{Zn}=x\\n_{Mg}=y\end{matrix}\right.\)
\(Zn+S\rightarrow\left(t^o\right)ZnS\)
x x ( mol )
\(Mg+S\rightarrow\left(t^o\right)MgS\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}65x+24y=23,4\\x+y=0,5\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=\dfrac{57}{205}\\y=\dfrac{91}{410}\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}m_{Zn}=\dfrac{57}{205}.65=\dfrac{741}{41}g\\m_{Mg}=\dfrac{91}{410}.24=\dfrac{1092}{205}g\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{741}{41}:23,4.100=77,23\%\\\%m_{Mg}=100\%-77,23\%=22,77\%\end{matrix}\right.\)
b.\(ZnS+2HCl\rightarrow ZnCl_2+H_2S\)
57/205 57/205 ( mol )
\(MgS+2HCl\rightarrow MgCl_2+H_2S\)
91/410 91/410 ( mol )
\(V_{H_2S}=\left(\dfrac{57}{205}+\dfrac{91}{410}\right).22,4=11,2l\)










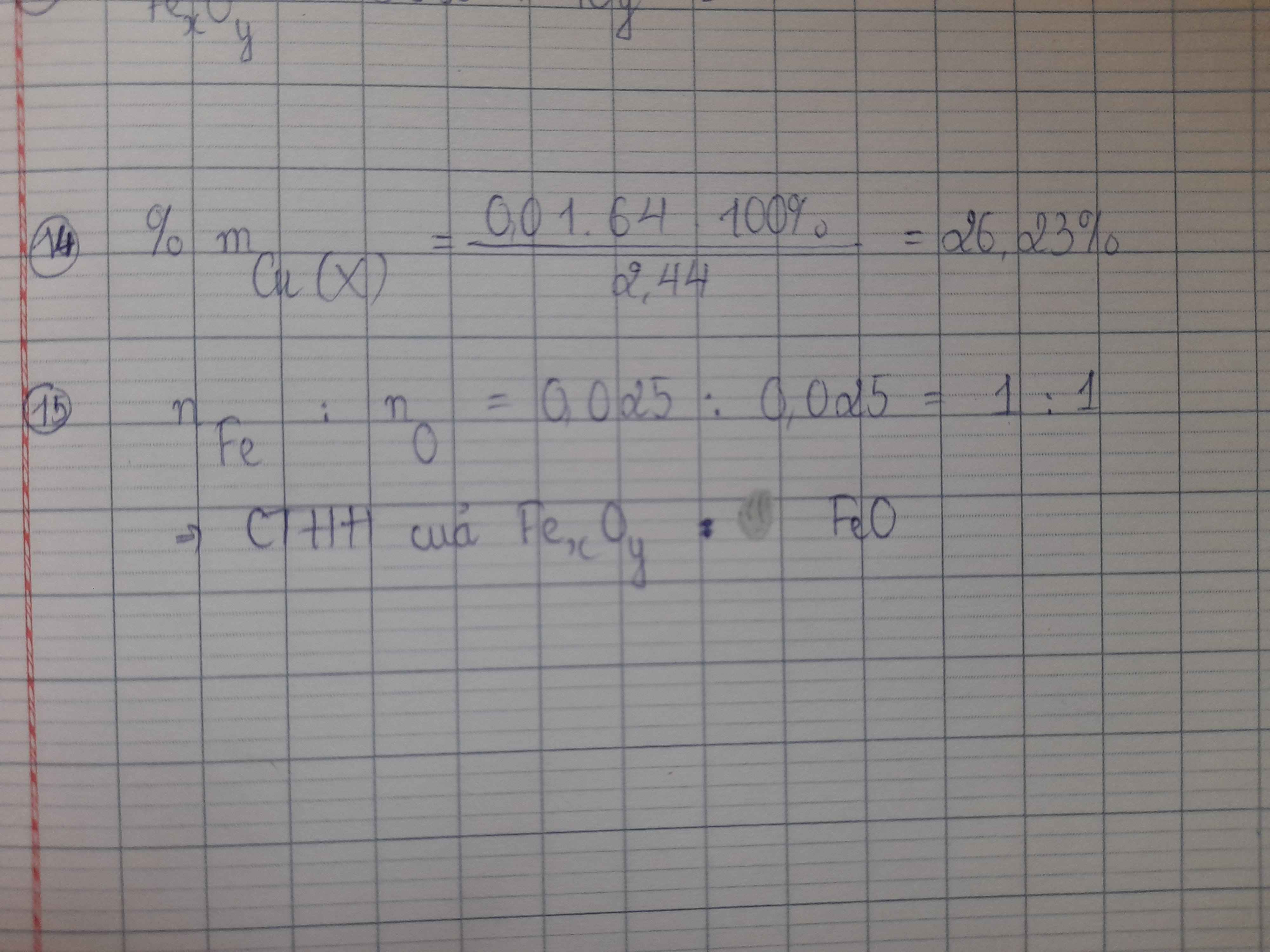



a)
nSO2=3,36 / 22,4=0,15 mol
Cu +2H2SO4 đ -t°-> CuSO4+SO2+2H2O
=> nCu = nSO2 = CuSO4 = 0,15 mol
mCuSO4=0,15.160=24g
mZnSO4=56,2-24=32,2g
nZnSO4=nZnO= 32,2/161=0,2 mol
m=mCu+mZnO=0,15.64+0,2.81=25,8g
b)
nH2SO4 pư=2nCu+nZnO=2.0,15+0,2=0,5 mol
nH2SO4 dư=0,5.10%=0,05mol
H2SO4+BaCl2 -> BaSO4+2HCl
nH2SO4dư=nBaSO4=0,05mol
mBaSO4=0,05.233=11,65g