Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(3Fe+2O_2-^{t^o}\rightarrow Fe_3O_4\)(1)
\(Fe_3O_4+8HCl\rightarrow FeCl_2+2FeCl_3+4H_2O\)(2)
\(Fe_{dư}+2HCl\rightarrow FeCl_2+H_2\)(3)
\(n_{Fe\left(dư\right)}=n_{H_2}=\frac{0,448}{22,4}=0,02\left(mol\right)\)
\(\Rightarrow m_{Fe\left(p.ứ\right)}=6,72-0,02.56=5,6\left(g\right)\)
\(\%m_{Fe\left(pứ\right)}=\frac{5,6}{6,72}.100=83,33\%\)
b) Muối trong dd B gồm FeCl2 và FeCl3
\(TheoPT\left(1\right):n_{Fe_3O_4}=\frac{1}{3}n_{Fe\left(pứ\right)}=\frac{1}{30}\left(mol\right)\)
\(TheoPT\left(2\right):n_{FeCl_2}=n_{Fe_3O_4}=\frac{1}{30}\left(mol\right)\)
\(TheoPT\left(2\right):n_{FeCl_3}=2n_{Fe_3O_4}=\frac{1}{15}\left(mol\right)\)
\(TheoPT\left(2\right):n_{FeCl_2}=n_{H_2}=0,02\left(mol\right)\)
\(\Rightarrow m_{FeCl_2}=\left(\frac{1}{30}+0,02\right).127=6,773\left(g\right)\)
\(\Rightarrow m_{FeCl_3}=\frac{1}{15}.162,5=10,833\left(g\right)\)

a,\(3Fe+2O_2\rightarrow Fe_3O_4\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(Fe_3O_4+8HCl\rightarrow2FeCl_3+FeCl_2+4H_2O\)
\(n_{H2}=\frac{0,448}{22,4}=0,02\left(mol\right)\)
\(\Rightarrow n_{Fe}=n_{H2}=0,02\left(mol\right)\)
\(\Rightarrow\%_{Fe}=\frac{6,72-0,02.56}{6,72}.100\%=83,33\%\)
b,\(n_{Fe3O4}=\frac{1}{30}\left(mol\right)\)
\(m_{FeCl3}=\frac{1}{15}.162,5=10,83\left(g\right)\)
\(\Rightarrow m_{FeCl2}=\left(\frac{1}{30}+0,02\right).127=6,773\left(g\right)\)

Gọi \(\left\{{}\begin{matrix}n_{Zn}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{1,33}{22,4}=0,059375\left(mol\right)\)
PTHH:
Zn + 2HCl ---> ZnCl2 + H2
a a
Fe + 2HCl ---> FeCl2 + H2
b b
Hệ pt \(\left\{{}\begin{matrix}65a+56b=3,73\\a+b=0,059375\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,045\left(mol\right)\\b=0,014375\left(mol\right)\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}m_{Zn}=0,045.65=2.925\left(g\right)\\m_{Fe}=0,014375.56=0,805\left(g\right)\end{matrix}\right.\\ \rightarrow\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{2,925}{3,73}=78,42\%\\\%m_{Fe}=100\%-78,42\%=21,58\%\end{matrix}\right.\)

a) PTHH: Fe + S --to--> FeS
Zn + S --to--> ZnS
FeS + 2HCl --> FeCl2 + H2S
ZnS + 2HCl --> ZnCl2 + H2S
Fe + 2HCl --> FeCl2 + H2
Zn + 2HCl --> ZnCl2 + H2
b)
Gọi số mol Zn, Fe là a, b (mol)
=> 65a + 56b = 3,72 (1)
Theo PTHH: \(a+b=n_{khí}=\dfrac{1,344}{22,4}=0,06\left(mol\right)\) (2)
(1)(2) => a = 0,04 (mol); b = 0,02 (mol)
=> \(\left\{{}\begin{matrix}m_{Zn}=0,04.65=2,6\left(g\right)\\m_{Fe}=0,02.56=1,12\left(g\right)\end{matrix}\right.\)
c) \(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{2,6}{3,72}.100\%=69,9\%\\\%m_{Fe}=\dfrac{1,12}{3,72}.100\%=30,1\%\end{matrix}\right.\)
Gọi \(\left\{{}\begin{matrix}n_{Zn}=x\\n_{Fe}=y\end{matrix}\right.\)
\(Zn+S\rightarrow\left(t^o\right)ZnS\)
x x x ( mol )
\(Fe+S\rightarrow\left(t^o\right)FeS\)
y y y ( mol )
\(n_{H_2S}=\dfrac{1,344}{22,4}=0,06mol\)
\(ZnS+2HCl\rightarrow ZnCl_2+H_2S\)
x x ( mol )
\(FeS+2HCl\rightarrow FeCl_2+H_2S\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}97x+88y=3,72+32\left(x+y\right)\\x+y=0,06\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}97x+88y=5,64\\x+y=0,06\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}x=0,04\\y=0,02\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}m_{Zn}=0,04.65=2,6g\\m_{Fe}=0,02.56=1,12g\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{2,6}{3,72}.100=69,89\%\\\%m_{Fe}=100\%-69,89\%=30,11\%\end{matrix}\right.\)


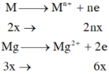
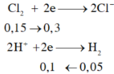
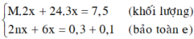
\(3Fe+2O_2\rightarrow Fe_3O_4\)
\(n_{Fe}=\frac{6,72}{22,4}=0,12\left(mol\right)\)
Cho hỗn hợp A tác dụng với HCl.
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(Fe_3O_4+8HCl\rightarrow FeCl_2+2FeCl_3+4H_2O\)
\(\%n_{Fe_{chuyen.hoa}}=\frac{0,1}{0,12}=83,33\%\)
\(n_{Fe3O4}=\frac{1}{2}n_{Fe_{pư}}=\frac{0,1}{3}\Rightarrow n_{FeCl3}=\frac{0,2}{3}\left(mol\right)\)
\(\Rightarrow n_{FeCl2}=0,12-\frac{0,2}{3}=\frac{4}{75}\left(mol\right)\)
\(\Rightarrow m_{FeCl3}=\frac{0,2}{3}.\left(56+35,5.3\right)=10,833\left(g\right)\)
\(\Rightarrow m_{FeCL2}=\frac{4}{75}.\left(56+35,5.2\right)=6,7733\left(g\right)\)