Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(CH_4+2O_2\underrightarrow{t^o}CO_2+H_2O\)
\(2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\)
Ta có: \(n_{CH_4}+n_{C_2H_2}=\dfrac{33,6}{22,4}=1,5\left(mol\right)\left(1\right)\)
Theo PT: \(n_{CO_2}=n_{CH_4}+2n_{C_2H_2}=\dfrac{56}{22,4}=2,5\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{CH_4}=0,5\left(mol\right)\\n_{C_2H_2}=1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,5.22,4}{33,6}.100\%\approx33,33\%\\\%V_{C_2H_2}\approx66,67\%\end{matrix}\right.\)
b, Theo PT: \(n_{O_2}=2n_{CH_4}+\dfrac{5}{2}n_{C_2H_2}=3,5\left(mol\right)\Rightarrow m_{O_2}=3,5.32=112\left(g\right)\)

Gọi \(\left\{{}\begin{matrix}n_{CO}=a\left(mol\right)\\n_{C_nH_{2n+2}}=b\left(mol\right)\end{matrix}\right.\)
=> \(a+b=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Bảo toàn C: \(a+bn=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
Bảo toàn H: \(2bn+2b=\dfrac{2,7}{18}.2=0,3\left(mol\right)\)
=> a = 0,15; b = 0,05; n = 2
=> CTPT: C2H6
\(\left\{{}\begin{matrix}\%V_{C_2H_6}=\dfrac{0,05}{0,2}.100\%=25\%\\\%V_{CO}=\dfrac{0,15}{0,2}.100\%=75\%\end{matrix}\right.\)

a. \(n_X=\dfrac{V_{O_2}}{22,4}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
\(n_{CO_2}=\dfrac{V_{O_2}}{22,4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
+ Bảo toàn C:
\(\Rightarrow n_{CH_4}+2n_{C_2H_4}=0,3\left(mol\right)\)
Mà: \(n_{CH_4}+n_{C_2H_4}=0,2\left(mol\right)\)
\(\Rightarrow0,2+n_{C_2H_4}=0,3\)
\(\Leftrightarrow n_{C_2H_4}=0,3-0,2=0,1\left(mol\right)\)
Phần trăm theo thể tích từng khí X là:
\(\%V_{C_2H_4}=\dfrac{0,1.100\%}{0,2}=50\%\)
\(\%V_{C_2H_4}=100\%-50\%=50\%\)
b. Bảo toàn H
\(\Rightarrow n_H=4n_{CH_4}+4n_{C_2H_4}\)
\(\Leftrightarrow n_H=4\left(n_{CH_4}+n_{C_2H_4}\right)\)
\(\Leftrightarrow n_H=4\left(0,1+0,1\right)\)
\(\Leftrightarrow n_H=4.0,2=0,8\left(mol\right)\)
\(\Rightarrow n_{H_2O}=\dfrac{n_H}{2}=\dfrac{0,8}{2}=0,4\left(mol\right)\)
Khối lượng nước thu được:
\(\Rightarrow m_{H_2O}=n_{H_2O}.M_{H_2O}=0,4.18=7,2\left(g\right)\)

TN2: C3H4 + AgNO3 + NH3 = C3H3Ag + NH4NO3
nC3H3Ag= 0.15 mol Từ PTHH: nC3H4= 0.15 mol mC3H4= 6g TN1: nCO2= 0.85 mol C3H4 + 4O2 -to-> 3CO2 + 2H2O 0.15____________0.45 C2H4 + 3O2 -to-> 2CO2 + 2H2O 0.2_____________0.85-0.45 mC2H4= 5.6g %C3H4= 6/(6+5.6)*100%= 51.72% %C2H2= 48.28%
\(2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\)
x x
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
y 2y
Gọi x là số mol của C2H2 (x,y>0)
y là số mol của C2H4
Ta có : \(22,4x+22,4y=13,44\)
\(n_{H_2O}=\dfrac{18}{18}=1\left(mol\right)\)
\(n_{H_2O}=2x+y=1\) ( Theo PTHH )
Ta có hệ PT :
\(\left\{{}\begin{matrix}22,4x+22,4y=13,44\\x+2y=1\end{matrix}\right.\)
Giải hệ PT , ta có :
x = 0,2
y = 0,4
\(V_{C_2H_2}=0,2.22,4=4,48\left(l\right)\)
\(\%V_{C_2H_2}=\dfrac{4,48}{13,44}.100\%\approx33,33\%\)
\(\%V_{C_2H_4}=\dfrac{0,4.22,4}{13,44}.100\%\approx66,67\%\)

nhh = V/22.4 = 4.48/22.4 = 0.2 (mol) = nCH4 + nC2H4
C2H4 + Br2 => C2H4Br2
nBr2 = m/M = 8/160 = 0.05 (mol)
VBr2 = 22.4 x 0.05 = 1.12 (l)
Theo pt --> nC2H4Br2 = 0.05 (mol)
mC2H4Br2 = n.M = 188x0.05 = 9.4 (g)
Theo pt ==> nC2H4 = 0.05 (mol)
===> nCH4 = 0.2 -0.05 = 0.15 (mol)
CH4 + 2O2 => CO2 + 2H2O
C2H4 + 3O2 => 2CO2 + 2H2O
nCH4 = 0.15 (mol) ==> nCO2 = 0.15 (mol)
nC2H4 = 0.05 (mol) ==> nCO2 = 0.1 (mol)
nCO2 = 0.25 (mol) ==> VCO2 = 22.4 x 0.25 = 5.6 (l)

a)
\(n_{Br_2}=\dfrac{8}{160}=0,05\left(mol\right)\)
PTHH: C2H4 + Br2 --> C2H4Br2
0,05<-0,05
=> \(n_{CH_4}=\dfrac{3,36}{22,4}-0,05=0,1\left(mol\right)\)
\(\%m_{CH_4}=\dfrac{0,1.16}{0,1.16+0,05.28}.100\%=53,33\%\)
\(\%m_{C_2H_4}=\dfrac{0,05.28}{0,1.16+0,05.28}.100\%=46,67\%\)
b)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,1-->0,2
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,05--->0,15
=> \(V_{O_2}=\left(0,2+0,15\right).22,4=7,84\left(l\right)\)
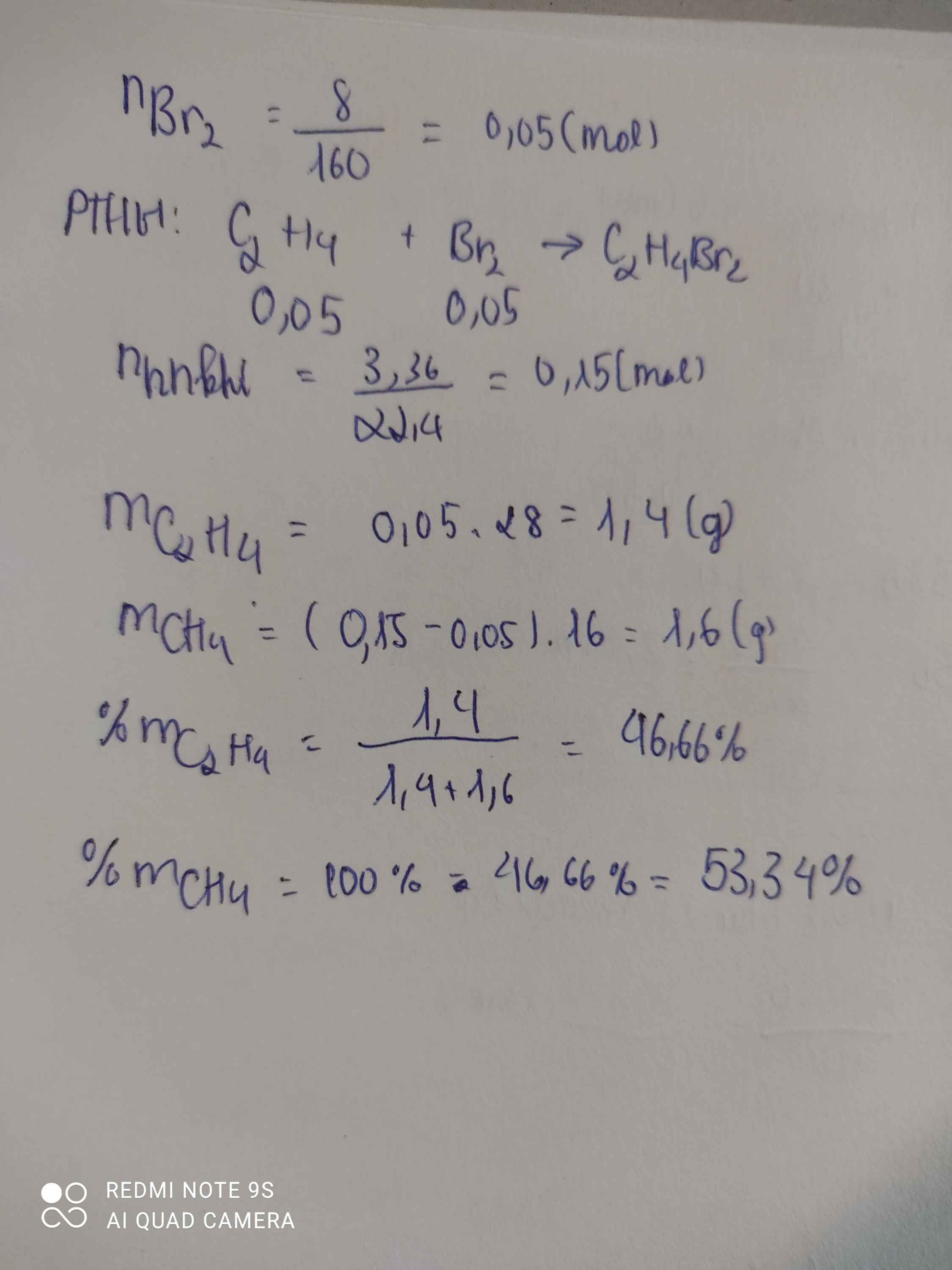
Sai đề bạn nhé !