Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, PT: \(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
Ta có: \(n_{C_2H_4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Theo PT: \(n_{O_2}=3n_{C_2H_4}=0,45\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,45.22,4=10,08\left(l\right)\)
b, Theo PT: \(n_{CO_2}=2n_{C_2H_4}=0,3\left(mol\right)\)
\(\Rightarrow V_{CO_2}=0,3.22,4=6,72\left(l\right)\)
c, PT: \(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_{3\downarrow}+H_2O\)
Theo PT: \(n_{CaCO_3}=n_{CO_2}=0,3\left(mol\right)\)
\(\Rightarrow m_{\downarrow}=m_{CaCO_3}=0,3.100=30\left(g\right)\)
Bạn tham khảo nhé!

a) PTHH : Mg + 2HCl \(\rightarrow\) MgCl2 + H2\(\uparrow\) (1)
MgO + 2HCl \(\rightarrow\) MgCl2 + H2O(2)
b) Theo đề cho : nH2=4.48/22.4=0.2(mol)
Theo PT(1): nMg=nH2=0.2(mol)
Do đó mMg(A)=0.2 \(\times\)24 =4.8(g)
mMgO(A) = 8.8-4,8=4(g)
c) Ta có : nMgO = 4/40 =0.1(mol)
Theo các PT(1)(2):
\(\Sigma\)nHCl(p/ư) = 2 \(\times\)(0.2 +0.1) =0.6(mol)
\(\Rightarrow\)VHCl = \(\dfrac{0.6}{2}\)=0.3(lít)

a)
$C_2H_5OH + 3O_2 \xrightarrow{t^o} 2CO_2 + 3H_2O$
b)
n C2H5OH = 9,2/46 = 0,2(mol)
n CO2 = 2n C2H5OH = 0,4(mol) => m CO2 = 0,4.44 = 17,6 gam
n H2O = 3n C2H5OH = 0,6(mol) => m H2O = 0,6.18 = 10,8 gam
c)
n O2 = 3n C2H5OH = 0,6(mol)
=> V O2 = 0,6.22,4 = 13,44(lít)
=> V không khí = 13,44/20% = 67,2 lít
Theo gt ta có: $n_{C_2H_5OH}=0,2(mol)$
a, $C_2H_5OH+3O_2\rightarrow 2CO_2+3H_2O$
b, Ta có: $n_{CO_2}=0,4(mol)\Rightarrow m_{CO_2}=17,6(g)$
$n_{H_2O}=0,6(mol)\Rightarrow m_{H_2O}=10,8(g)$
c, Ta có: $n_{O_2}=0,6(mol)\Rightarrow V_{O_2}=13,44(l)\Rightarrow V_{kk}=67,2(l)$

a, \(CH_4+2O_2\underrightarrow{^{t^o}}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{^{t^o}}2CO_2+2H_2O\)
b, Gọi: \(\left\{{}\begin{matrix}n_{CH_4}=x\left(mol\right)\\n_{C_2H_4}=y\left(mol\right)\end{matrix}\right.\) \(\Rightarrow x+y=\dfrac{4,48}{22,4}=0,2\left(mol\right)\left(1\right)\)
Theo PT: \(n_{O_2}=2n_{CH_4}+3n_{C_2H_4}=2x+3y=\dfrac{15,68}{22,4}=0,7\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=-0,1\\y=0,3\end{matrix}\right.\)
Đến đây thì ra số mol âm, bạn xem lại đề nhé.

a. \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
a 2a a
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
b 3b 2b
b. \(n_{O_2}=\dfrac{25.88}{22.4}=1.155mol\)
n hỗn hợp khí \(=\dfrac{11.2}{22.4}=0.5mol\)
Ta có: \(\left\{{}\begin{matrix}a+b=0.5\\2a+3b=1.155\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0.345\\b=0.155\end{matrix}\right.\)
\(\%V_{CH_4}=\dfrac{0.345\times22.4\times100}{11.2}=69\%\)
\(\%V_{C_2H_4}=100-69=31\%\)
c. \(V_{CO_2}=\left(a+2b\right)\times22.4=\left(0.345+2\times0.155\right)\times22.4=14.672l\)

a)
\(n_{KClO_3}=\dfrac{24.5}{122.5}=0.2\left(mol\right)\)
\(2KClO_3\underrightarrow{^{^{t^0}}}2KCl+3O_2\)
\(n_{O_2}=\dfrac{3}{2}\cdot0.2=0.3\left(mol\right)\)
\(V_{O_2}=0.3\cdot22.4=6.72\left(l\right)\)
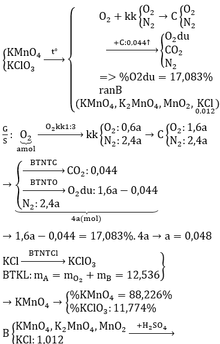
\(n_{O_2}=\dfrac{2.24}{22.4}=0.1\left(mol\right)\)
\(2KMnO_4\underrightarrow{^{^{t^0}}}K_2MnO_4+MnO_2+O_2\)
\(0.2...............................................0.1\)
\(n_{KMnO_4\left(bđ\right)}=\dfrac{0.2}{90\%}=\dfrac{2}{9}\left(mol\right)\)
\(m_{KMnO_4}=\dfrac{2}{9}\cdot158=35.11\left(g\right)\)
a, PT: \(S+O_2\underrightarrow{t^o}SO_2\)
\(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
b, Ta có: \(n_{SO_2}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
Theo PT: \(n_S=n_{SO_2}=0,1\left(mol\right)\)
⇒ mP = 6,3 - mS = 6,3 - 0,1.32 = 3,1 (g)
\(\Rightarrow n_P=\dfrac{3,1}{31}=0,1\left(mol\right)\)
Theo PT: \(n_{O_2}=n_S+\dfrac{5}{4}n_P=0,225\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,225.24,79=5,57775\left(l\right)\)
c, Theo PT: \(n_{P_2O_5}=\dfrac{1}{2}n_P=0,05\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=0,05.142=7,1\left(g\right)\)