Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(2KMnO_4\underrightarrow{t^O}K_2MnO_4+MnO_2+O_2\)
\(n_{O_2}=\dfrac{3,2}{32}=0,1mol\)
Theo pt \(\Rightarrow n_{KMnO_4}=2n_{O_2}=2\cdot0,1=0,2mol\)
\(\Rightarrow m=31,6g\)
b)\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
\(n_{O_2}=\dfrac{7,437}{22,4}=0,33mol\)
\(\Rightarrow n_{KClO_3}=\dfrac{3}{2}n_{O_2}=0,5mol\)
\(\Rightarrow m=61,25g\)
c) Cùng 1 số mol , kali clorat sẽ cho nhiều oxi sản phẩm hơn

Câu 6.
\(n_{O_2}=\dfrac{16,8}{22,4}=0,75mol\)
\(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
1,5 0,75
\(m_{KMnO_4}=1,5\cdot158=237g\)
Câu 7.
\(n_{Fe_3O_4}=\dfrac{4,64}{232}=0,02mol\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
0,04 0,02
\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
\(\dfrac{2}{75}\) 0,04
\(m_{KClO_3}=\dfrac{2}{75}\cdot122,5=\dfrac{49}{15}\approx3,27g\)

a, PT: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Ta có: \(n_{KMnO_4}=\dfrac{31,6}{158}=0,2\left(mol\right)\)
Theo PT: \(n_{K_2MnO_4}=\dfrac{1}{2}n_{KMnO_4}=0,1\left(mol\right)\)
\(\Rightarrow m_{K_2MnO_4}=0,1.197=19,7\left(g\right)\)
b, Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{KMnO_4}=0,1\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,1.24,79=2,479\left(l\right)\)
c, PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
Theo PT: \(\left\{{}\begin{matrix}n_{CO_2}=\dfrac{1}{2}n_{O_2}=0,05\left(mol\right)\\n_{H_2O}=n_{O_2}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow V_{CO_2}=0,05.24,79=1,2395\left(l\right)\)
\(m_{H_2O}=0,1.18=1,8\left(g\right)\)

\(n_{H_2}=\dfrac{V}{24,79}=\dfrac{11,2}{24,79}\approx0,45\left(mol\right)\)
a) \(PTHH:2H_2+O_2\underrightarrow{t^o}2H_2O\)
2 1 2
0,45 0,225 0,45
b) \(m_{O_2}=n.M=0,225.\left(16.2\right)=7,2\left(g\right)\\ V_{O_2}=n.24,79=0,225.24,79=5,57775\left(l\right)\)
c) \(PTHH:2KMnO_4\rightarrow K_2MnO_4+MnO_2+O_2\)
2 1 1 1
0,45 0,225 0,225 0,225
\(m_{KMnO_4}=n.M=0,45.\left(39+55+16.4\right)=71,1\left(g\right).\)
a, \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
b, Ta có: \(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=0,25\left(mol\right)\)
\(\Rightarrow m_{O_2}=0,25.32=8\left(g\right)\)
\(V_{O_2}=0,25.22,4=5,6\left(l\right)\)
c, \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Theo PT: \(n_{KMnO_4}=2n_{O_2}=0,5\left(mol\right)\Rightarrow m_{KMnO_4}=0,5.158=79\left(g\right)\)

\(n_{O_2\left(dktc\right)}=\dfrac{V}{22,4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ PTHH:2KMnO_4-^{t^o}>K_2MnO_4+MnO_2+O_2\)
tỉ lệ 2 ; 1 ; 1 : 1
n(mol) 0,3<------------------0,15<---------0,15<-----0,15
\(m_{KMnO_4}=n\cdot M=0,3\cdot\left(39+55+16\cdot4\right)=47,4\left(g\right)\)

Nhiệt phân hoàn toàn 31,6 gam KMnO4 để điều chế oxi. Thể tích khí O2 thu được ở đktc là:
(K = 39; Mn = 55; O = 16)
A.
8,96 lít
B.
4,48 lít
C.
1,12 lít
D.
2,24 lít

PT: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
Ta có: \(n_{Al}=\dfrac{3,24}{27}=0,12\left(mol\right)\)
a, \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,09\left(mol\right)\) \(\Rightarrow V_{O_2}=0,09.22,4=2,016\left(l\right)\)
b, \(n_{Al_2O_3}=\dfrac{1}{2}n_{Al}=0,06\left(mol\right)\) \(\Rightarrow m_{Al_2O_3}=0,06.102=6,12\left(g\right)\)
c, \(V_{kk}=\dfrac{2,016}{21\%}=9,6\left(l\right)\)
d, PT: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Theo PT: \(n_{KMnO_4}=2n_{O_2}=0,18\left(mol\right)\)
\(\Rightarrow m_{KMnO_4}=0,18.158=28,44\left(g\right)\)

Bạn xem lời giải ở đây nhé.
https://hoc24.vn/cau-hoi/cho-324-g-al-tac-dung-voi-oxi-vua-du-th-duoc-al2o3-a-tinh-vo2-b-tinh-m-al2o3-c-trong-vkk-can-dung-biet-vo2-21-vkk-d-tinh-khoi-luong-kmno.7651142171785
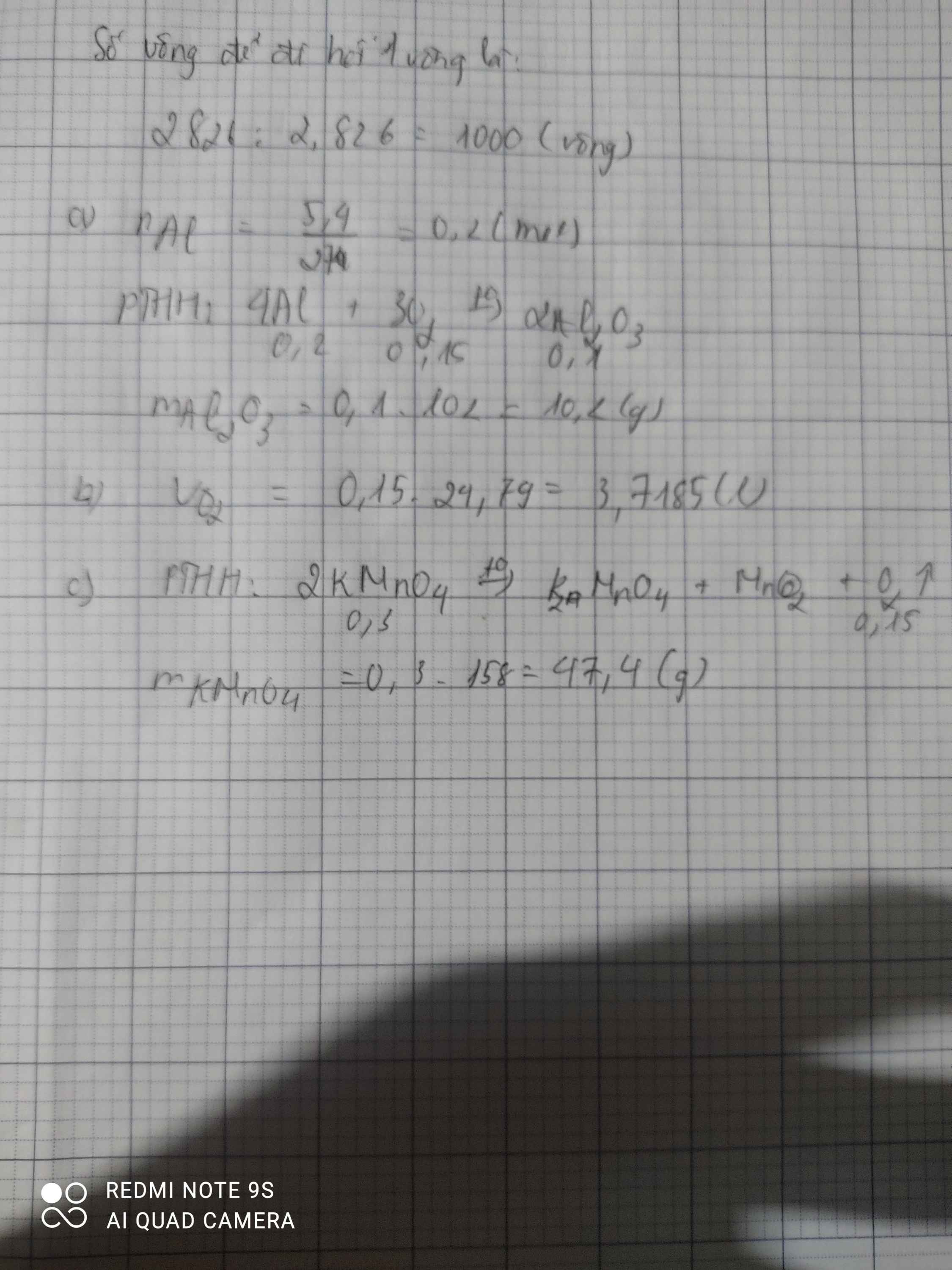
\(2KMnO_4\xrightarrow[]{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
\(n_{O_2}=\dfrac{V_{O_2}}{24,79}=\dfrac{0,7437}{24,79}=0,03\left(mol\right)\)
Theo PTHH: \(n_{KMnO_4}=2n_{O_2}\Rightarrow n_{KMnO_4}=0,06\left(mol\right)\)
Khối lượng \(KMnO_4\) cần dùng:
\(m_{KMnO_4}=n_{KMnO_4}.M_{KMnO_4}=0,03.158=4,47\left(g\right)\)
\(n_{O_2}\)=0,7437/22,4\(\approx0,03\)(m)
PTHH : 2KMnO4 —> K2MnO4 + MnO2 + O2
tỉ lệ :2 1 1 1
số mol:0,06 0,03 0,03 0,03
\(m_{KMnO_4}\)=0,06.158=9,48(g)