Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Cl_2}=\dfrac{2,1168}{22,4}=0,0945\left(mol\right)\)
=> nCl(muối) = 0,39 - 0,0945 = 0,201 (mol)
=> nAgCl = 0,201 (mol)
=> mAgCl = 0,201.143,5 = 28,8435 (g)

\(Đặt:\)
\(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{6.72}{22.4}=0.3\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\)
\(m_{hh}=24x+56y=13.6\left(g\right)\\ n_{H_2}=x+y=0.3\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}x=0.1\\y=0.2\end{matrix}\right.\)
\(\%Mg=\dfrac{0.1\cdot24}{13.6}\cdot100\%=17.64\%\\ \%Fe=100-17.64=82.36\%\)
\(n_{HCl}=2n_{H_2}=2\cdot0.3=0.6\left(mol\right)\)
\(V_{HCl}=\dfrac{0.6}{2}=0.3\left(l\right)\)
\(m_Y=m_{MgCl_2}+m_{FeCl_2}=0.1\cdot95+0.2\cdot127=34.9\left(g\right)\)

Gọi :
\(n_{KMnO_4} = n_{K_2Cr_2O_7} = n_{K_2MnO_4} = n_{MnO_2} = n_{PbO_2} = x(mol)\)
Suy ra : 975x = m(1)
Bảo toàn nguyên tố với K,Mn,Cr,Pb ,Muối gồm :
\(\left\{{}\begin{matrix}KCl:x+2x+2x=5x\left(mol\right)\\MnCl_2:x+x+x=3x\left(mol\right)\\CrCl_3:2x\left(mol\right)\\PbCl_2:x\left(mol\right)\end{matrix}\right.\)
Suy ra : 1345,5x = m + 11,856(2)
Từ (1)(2) suy ra : m = 31,2 ; x = 0,032
Bảo toàn electron :
\(5n_{KMnO_4} + 6n_{K_2Cr_2O_7} + 4n_{K_2MnO_4} + 2n_{MnO_2} + 2n_{PbO_2} = 2n_{Cl_2}\)
Suy ra :
\(a = \dfrac{0,032.5+0,032.6 + 0,032.4 + 0,032.2 + 0,032.2}{2} = 0,304(mol)\)
(Đáp án B)

\(Đặt:n_{MnO_2}=a\left(mol\right),n_{KMnO_4}=b\left(mol\right)\)
\(m_{hh}=87a+158b=37.96\left(g\right)\left(1\right)\)
\(n_{Cl_2}=\dfrac{10.08}{22.4}=0.45\left(mol\right)\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(n_{Cl_2}=a+2.5b=0.45\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.4,b=0.02\)
\(\%MnO_2=\dfrac{0.4\cdot87}{37.96}\cdot100\%=91.68\%\\\%KMnO_4=100-91.68=8.32\% \)
\(m_M=m_{KCl}+m_{MnCl_2}=0.02\cdot74.5+\left(0.4+0.02\right)\cdot126=54.41g\)

Đáp án B
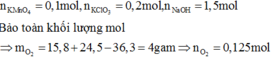
Khi cho hỗn hợp Y phản ứng với HCl đặc sẽ xảy ra phản ứng oxi hóa – khử tạo ra Cl2
![]()
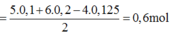


\(2KMnO_4+16HCl_{đặc,nóng}\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\\ MnO_2+4HCl_{đặc,nóng}\rightarrow MnCl_2+Cl_2+2H_2O\\ Đặt:n_{KMnO_4}=a\left(mol\right);n_{MnO_2}=b\left(mol\right)\left(a,b>0\right)\\ Vì:n_{Cl_2}=\dfrac{9,632}{22,4}=0,43\left(mol\right)\\ \Rightarrow2,5a+b=0,43\left(1\right)\\ Ta.có:n_O=4a+2b\Rightarrow m_O=16.\left(4a+2b\right)=64a+32b=0,39114.\left(158a+87b\right)\\ \Leftrightarrow64a+32b-61,80012a-34,02918b=0\\ \Leftrightarrow2,19988a-2,02918b=0\left(2\right)\\ \left(1\right),\left(2\right)\Rightarrow\left\{{}\begin{matrix}2,19988a-2,02918b=0\\2,5a+b=0,43\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,12\\b=0,13\end{matrix}\right.\)
\(n_{MnCl_2}=n_{MnO_2}=a+b=0,25\left(mol\right)\\ \Rightarrow m_{MnCl_2}=126.0,25=31,5\left(g\right)\)
=>Chọn D



Đáp án B