
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

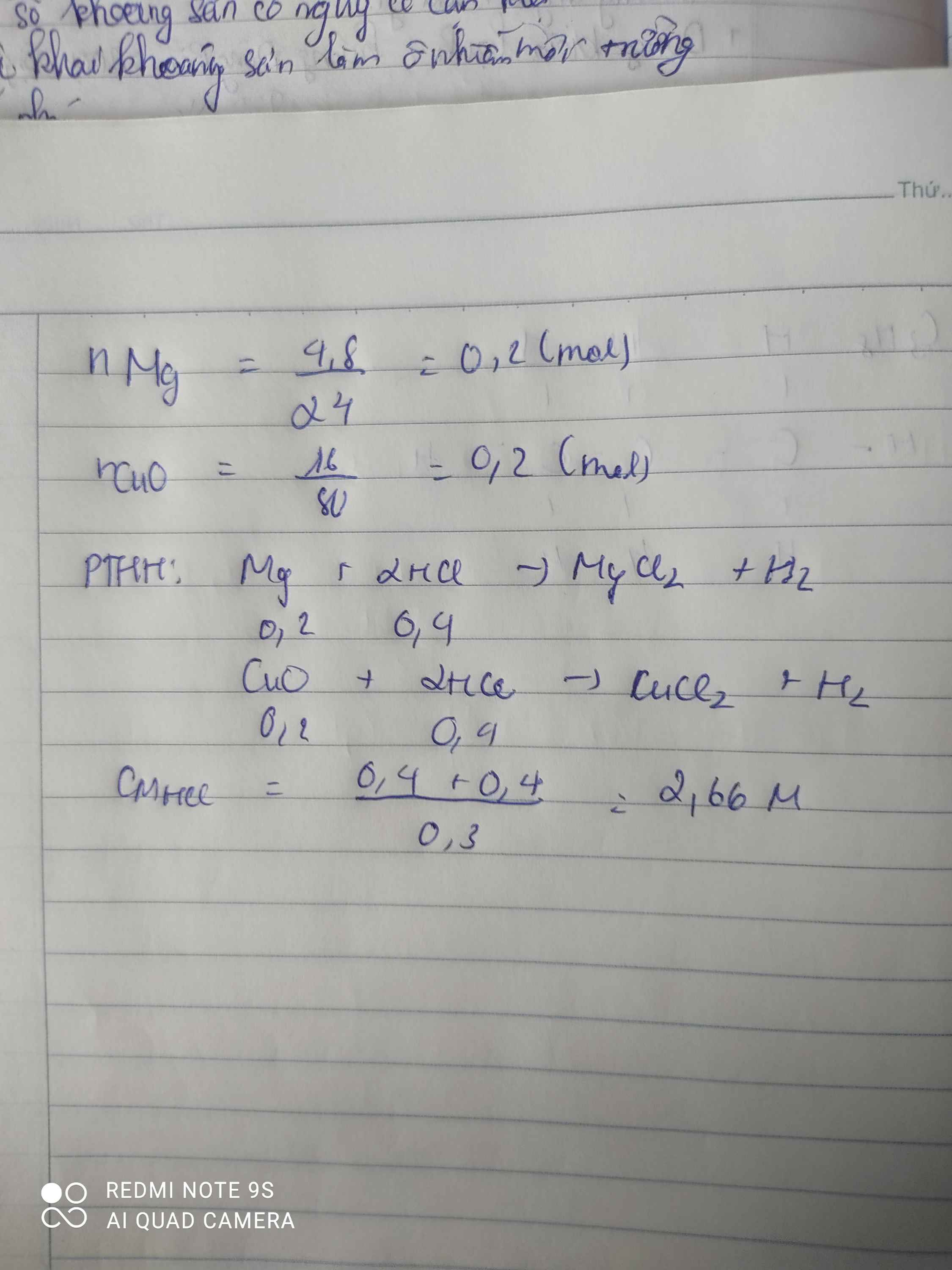

\(a,Fe+2HCl\rightarrow FeCl_2+H_2\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\Rightarrow n_{Fe}=n_{H_2}=0,1\left(mol\right)\\ \Rightarrow\%m_{Fe}=\dfrac{0,1.56}{13,6}.100\%\approx41,176\%\\ \Rightarrow\%m_{CuO}\approx58,824\%\\ b,n_{CuO}=\dfrac{13,6-0,1.56}{80}=0,1\left(mol\right)\\ n_{HCl\left(p.ứ\right)}=2.\left(n_{Fe}+n_{CuO}\right)=2.\left(0,1+0,1\right)=0,4\left(mol\right)\\ \Rightarrow V_{ddHCl}=\dfrac{0,4}{2}=0,2\left(l\right)\)

\(n_{HCl}=0.5\cdot1=0.5\left(mol\right)\)
\(n_{H_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(0.15.....0.3........................0.15\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(0.1.........0.5-0.3\)
\(m_A=0.15\cdot24+0.1\cdot80=11.6\left(g\right)\)

\(n_{H^+}=0,5.0,8+0,25.0,8.2=0,8\left(mol\right)\\ \Rightarrow n_{H_2}=\dfrac{n_{H^+}}{2}=\dfrac{0,8}{2}=0,4\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,4.22,4=8,96\left(l\right)\)
Không phải KL hhX cho rồi à ta?

a) Sửa đề: dd H2SO4 9,8%
Ta có: \(n_{H_2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\) \(\Rightarrow m_{H_2}=0,35\cdot2=0,7\left(g\right)\)
Bảo toàn nguyên tố: \(n_{H_2SO_4}=n_{H_2}=0,35\left(mol\right)\) \(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,35\cdot98}{9,8\%}=350\left(g\right)\)
\(\Rightarrow m_{dd}=m_{KL}+m_{H_2SO_4}-m_{H_2}=361,6\left(g\right)\)
b) Tương tự câu a

a, PT: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
Ta có: \(n_{H_2}=\dfrac{0,672}{22,4}=0,03\left(mol\right)\)
Theo PT: \(n_{Mg}=n_{H_2}=0,03\left(mol\right)\)
\(\Rightarrow m_{Mg}=0,03.24=0,72\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,72}{1,74}.100\%\approx41,38\%\\\%m_{AlCl_3}\approx58,62\%\end{matrix}\right.\)
b, Theo PT: \(n_{HCl}=2n_{H_2}=0,06\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,06.36,5=2,19\left(g\right)\)
\(\Rightarrow C\%_{ddHCl}=\dfrac{2,19}{500}.100\%=0,438\%\)
Bạn tham khảo nhé!
a, nH2 = 0,03 ( mol )
=> nMg = nH2 = 0,03 ( mol )
=> mMg = 0,72 g
=> %Mg \(\approx\) 41,38 % .
=> % Al \(\approx\) 58,62 % .
b, Có : nH2 = 0,03 mol
=> nHCl = nHCltừ Al2O3 + nHCltừ Mg = 0,06 + 0,06 = 0,12 ( mol )
=> mHCl = 4,38 ( g )
Lại có : mdd = mhh + mddHCl = 501,74 ( g )
=> \(C\%=\dfrac{m_{HCl}}{m_{dd}}.100\%\approx0,87\%\)
( chắc đoạn trên là Al2O3 :vvvv )

Câu 1:
Gọi : nMg=a(mol); nMgO=b(mol) (a,b>0)
a) PTHH: Mg + 2 HCl -> MgCl2 + H2
a________2a_______a______a(mol)
MgO +2 HCl -> MgCl2 + H2O
b_____2b_______b___b(mol)
Ta có hpt:
\(\left\{{}\begin{matrix}24a+40b=8,8\\22,4a=4,48\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\)
=> mMg=0,2.24=4,8(g)
=>%mMg= (4,8/8,8).100=54,545%
=> %mMgO= 45,455%
b) m(muối)=mMg2+ + mCl- = 0,3. 24 + 0,6.35,5=28,5(g)
c) V=VddHCl=(2a+2b)/2=0,3(l)=300(ml)
Câu 2:
Ta có: \(\left\{{}\begin{matrix}n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\n_{HCl}=0,4\cdot2=0,8\left(mol\right)\end{matrix}\right.\)
PTHH: \(Ca+2HCl\rightarrow CaCl_2+H_2\uparrow\)
0,2____0,4_____0,2____0,2 (mol)
\(CaO+2HCl\rightarrow CaCl_2+H_2O\)
0,2____0,4______0,2____0,2 (mol)
Ta có: \(\left\{{}\begin{matrix}\%m_{Ca}=\dfrac{0,2\cdot40}{0,2\cdot40+0,2\cdot56}\cdot100\%\approx41,67\%\\\%m_{CaO}=58,33\%\\m_{CaCl_2}=\left(0,2+0,2\right)\cdot111=44,4\left(g\right)\end{matrix}\right.\)

a) Gọi số mol Mg, CuO là a, b (mol)
=> 24a + 80b = 14 (1)
\(n_{HCl}=\dfrac{255,5.10\%}{36,5}=0,7\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
a--->2a--------->a------>a
CuO + 2HCl --> CuCl2 + H2O
b------>2b----->b
=> 2a + 2b = 0,7 (2)
(1)(2) => a = 0,25 (mol); b = 0,1 (mol)
=> \(\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,25.24}{14}.100\%=42,857\%\\\%m_{CuO}=\dfrac{0,1.80}{14}.100\%=57,143\%\end{matrix}\right.\)
b)
mdd sau pư = 14 + 255,5 - 0,25.2 = 269 (g)
\(C\%_{MgCl_2}=\dfrac{0,25.95}{269}.100\%=8,829\%\)
\(C\%_{CuCl_2}=\dfrac{0,1.135}{269}.100\%=5,019\%\)