Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
PTHH: 2K + 2H2O ---> 2KOH + H2
0,1---------------->0,1----->0,05
\(m_{ct}=m_{KOH}=0,1.56=5,6\left(g\right)\\ m_{dd}=m_K+m_{H_2O}-m_{H_2}=96,2+3,9-0,05.2=100\left(g\right)\)
\(C\%_{KOH}=\dfrac{5,6}{100}.100\%=5,6\%\\ b,m_{dd}=100+50=150\left(g\right)\\ C\%_{KOH}=\dfrac{5,6}{150}.100\%=3,37\%\)
c, Gọi \(m_{H_2O}=a\left(g\right)\)
\(\Rightarrow C\%_{KOH}=\dfrac{5,6}{100+a}.100\%=2,8\%\\ \Leftrightarrow a=100\left(g\right)\)
d, Gọi \(m_{KOH}=a\left(g\right)\)
\(\Rightarrow C\%_{KOH}=\dfrac{5,6+a}{100+a}.100\%=22,4\%\\ \Leftrightarrow a=21,65\left(g\right)\)

a)
\(n_{K_2O}=\dfrac{18,8}{94}=0,2\left(mol\right)\)
PTHH: K2O + H2O --> 2KOH
0,2-->0,2---->0,4
mct = 0,4.56 = 22,4 (g)
mdm = 81,2 - 0,2.18 = 77,6 (g)
mdd = 22,4 + 77,6 = 100 (g)
b)
\(C\%=\dfrac{22,4}{100}.100\%=22,4\%\)
c)
\(C\%=\dfrac{22,4}{50+100}.100\%=14,933\%\)
d)
\(m_{dd\left(sau.khi.thêm\right)}=\dfrac{22,4.100}{11,2}=200\left(g\right)\)
=> mH2O(thêm) = 200 - 100 = 100 (g)
e) Gọi khối lượng KOH thêm là x (g)
Có: \(C\%_{\left(dd.sau.khi.thêm\right)}=\dfrac{22,4+x}{100+x}.100\%=30\%\)
=> x = 10,857 (g)

\(n_K=\dfrac{31,2}{39}=0,8\left(mol\right)\)
PTHH :
\(2K+2H_2O\underrightarrow{t^o}2KOH+H_2\uparrow\)
0,8 0,8 0,4
\(a,V_{H_2}=0,4.22,4=8,96\left(l\right)\)
\(b,m_{KOH}=0,8.56=44,8\left(g\right)\)
\(m_{ddKOH}=\left(31,2+200\right)-\left(0,4.2\right)=300,4\left(g\right)\)
\(c,C\%_{KOH}=\dfrac{44,8}{\left(200+31,2\right)-\left(0,4.2\right)}.100\%\approx19,44\%\)

2Na + 2H2O \(\rightarrow\) 2NaOH + H2 (1)
K2O + H2O \(\rightarrow\) 2KOH (2)
Có : mdd B = mhh A + mH2O - mH2 = 19,85 + 180,4 - mH2 = 200
\(\Rightarrow\) mH2 = 0,25(g)
\(\Rightarrow\) nH2 = 0,25/2 = 0,125(mol)
Theo PT(1) \(\Rightarrow\)nNa = nNaOH = 2.nH2 = 2. 0,125 = 0,25(mol)
\(\Rightarrow\left\{{}\begin{matrix}m_{Na}=0,25.23=5,75\left(g\right)\\m_{NaOH}=0,25.40=10\left(g\right)\end{matrix}\right.\)
\(\Rightarrow\) mK2O = 19,85 - 5,75= 14,1(g)
\(\Rightarrow\) nK2O = 14,1/94 = 0,15(mol)
Theo PT(2) \(\Rightarrow\) nKOH = 2 . nK2O = 2. 0,15 = 0,3(mol)
\(\Rightarrow\) mKOH = 0,3 . 56 = 16,8(g)
* C%KOH / ddB = 16,8/200 . 100% = 8,4%
C%NaOH / dd B = 10/200 . 100% = 5%
* m(KOH+ NaOH) = 16 ,8 + 10 =\ 26,8(g)
\(\Rightarrow\)mH2O / dd B = 200 - 26,8 = 173,2 (g)
\(\Rightarrow\) VH2O / dd B = m : D = 173,2 : 1 = 173,2 (ml) =0,1732(l)
mà Vdd B = VH2O / ddB
=> Vdd B =\ 0,1732(l)
Do đó :
CM của NaOH / dd B = 0,25/0,1732=1,44(M)
CM của KOH / dd B = 0,3/0,1732 = 1,73 (M)

$2K + 2H_2O \to 2KOH + H_2$
$n_{KOH} = n_K = \dfrac{21,06}{39} = 0,54(mol)$
Sau khi hòa tan :
$n_{KOH} = 0,54 + 0,4 = 0,94(mol)$
$C_{M_{KOH}} = \dfrac{0,94}{0,4} = 2,35M$
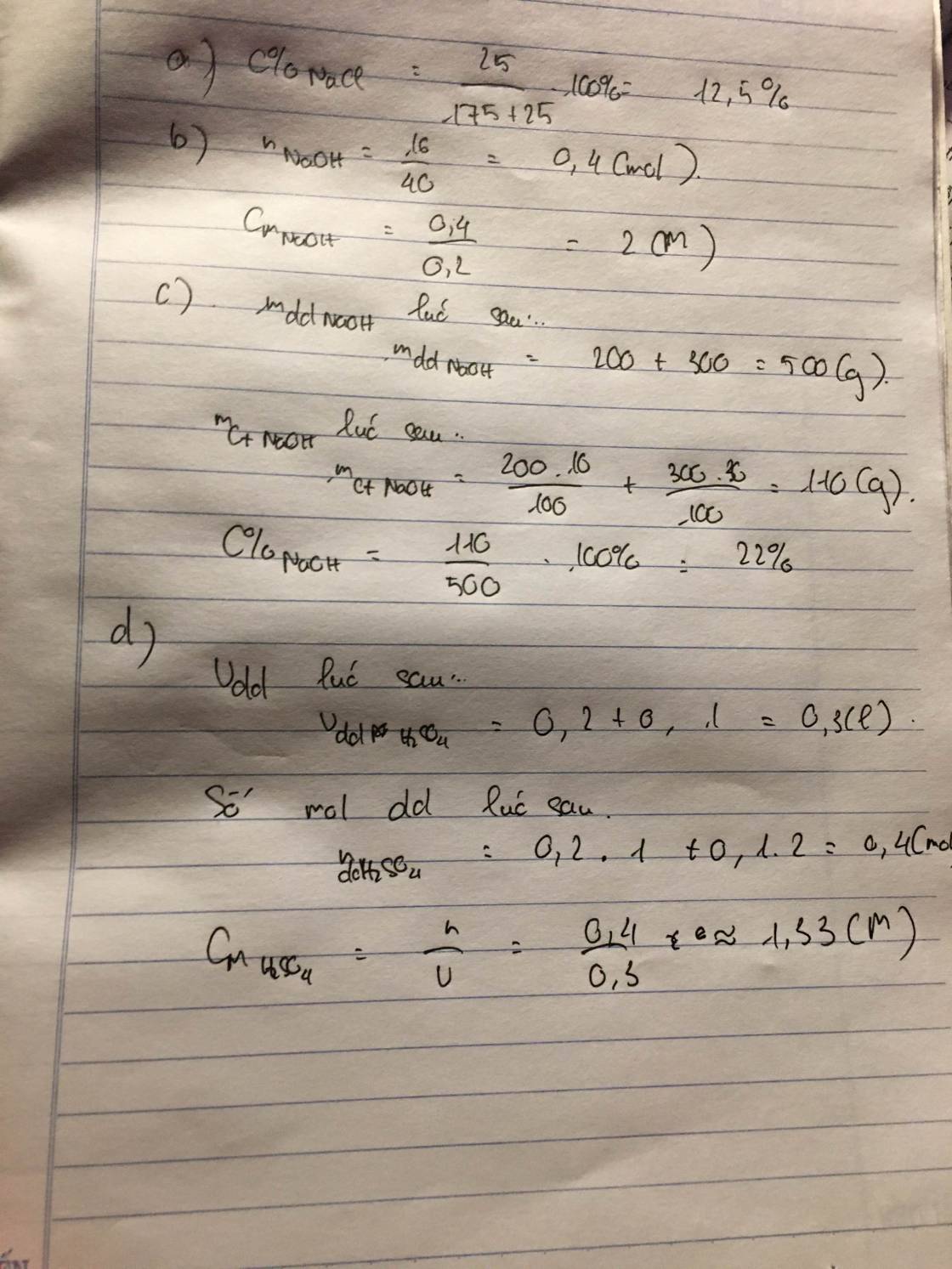
$2K + 2H_2O \to 2KOH + H_2$
$n_{KOH} = n_K = \dfrac{9,36}{39} =0,24(mol)$
$n_{H_2} = \dfrac{1}{2}n_K = 0,12(mol)$
$m_{dd\ sau\ pư} = 9,36 + 70,88 - 0,12.2 = 80(gam)$
$C\%_{KOH} = \dfrac{0,24.56}{80}.100\% = 16,8\%$