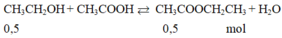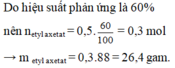Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{C2H5OH}=\dfrac{34,5}{46}=0,75\left(mol\right)\)
Pt : \(C_2H_5OH+3O_2\xrightarrow[]{t^o}2CO_2+3H_2O\)
0,75 1,5
\(CH_3COOH+C_2H_5OH⇌CH_3COOC_2H_5+H_2O\)
0,75 0,75 0,75
a) \(V_{CO2\left(dktc\right)}=1,5.22,4=33,6\left(l\right)\)
b) \(m_{CH3COOC2H5\left(lt\right)}=0,75.88=66\left(g\right)\)
⇒\(m_{CH3COOC2H5\left(tt\right)}=\) \(66.90\%=59,4\left(g\right)\)
Chúc bạn học tốt
À , ý b) trong lúc làm bài bạn bổ sung vào giúp mình nhé

Bài 1:
PTHH: \(C_2H_5OH+O_2\xrightarrow[]{mengiấm}CH_3COOH+H_2O\)
Ta có: \(n_{C_2H_5OH}=\dfrac{115\cdot0,8}{46}=2\left(mol\right)=n_{CH_3COOH\left(lýthuyết\right)}\)
\(\Rightarrow m_{CH_3COOH\left(thực\right)}=2\cdot60\cdot90\%=108\left(g\right)\)
Bài 2:
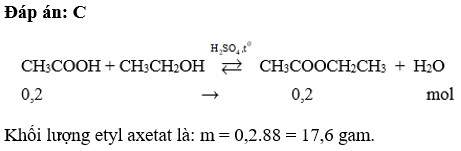
PTHH: \(C_2H_5OH+CH_3COOH\xrightarrow[H_2SO_4\left(đ\right)]{t^o}CH_3COOC_2H_5+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{CH_3COOH}=\dfrac{60}{60}=1\left(mol\right)\\n_{C_2H_5OH}=\dfrac{92}{46}=2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Rượu còn dư, Axit p/ứ hết
\(\Rightarrow n_{CH_3COOC_2H_5\left(lýthuyết\right)}=1\left(mol\right)\) \(\Rightarrow m_{CH_3COOC_2H_5\left(thực\right)}=1\cdot88\cdot80\%=70,4\left(g\right)\)

$PTHH : CH_3COOH + C_2H_5OH \buildrel{{H_2SO_4 đ,t^o}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O \\ n_{CH_3COOH} = \dfrac{3}{60} = 0,05(mol) \\ n_{C_2H_5OH} = \dfrac{2,5}{46} = 0,054(mol) \\ Ta có : n_{C_2H_5OH} > n_{CH_3COOH} \to C_2H_5OH dư \\ n_{ CH_3COOC_2H_5 } = n_{CH_3COOH} = 0,05(mol) \\ m_{este} = 0,05.88 = 4,4(gam) \\ m_{este(tt)} = 4,4.0,9 = 3,96(gam)$

\(a)n_{CH_3COOH} = 0,2.2 = 0,4(mol)\\ Mg + 2CH_3COOH \to (CH_3COO)_2Mg + H_2\\ n_{Mg} = \dfrac{1}{2}n_{CH_3COOH} = 0,2(mol)\\ m_{Mg} = 0,2.24 = 4,8(gam)\\ b)\\ CH_3COOH + C_2H_5OH \buildrel{{H_2SO_4}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O\\ n_{CH_3COOH\ pư} = n_{este} = \dfrac{24,64}{88} = 0,28(mol)\\ H = \dfrac{0,28}{0,4}.100\% = 70\%\)

\(CaCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Ca+CO_2+H_2O\\ a,n_{CH_3COOH}=0,2.1=0,2\left(mol\right)\Rightarrow n_{CO_2}=\dfrac{0,2}{2}=0,1\left(mol\right)\Rightarrow V_{CO_2\left(đktc\right)}=0,1.22,4=2,24\left(l\right)\\ b,CH_3COOH+C_2H_5OH⇌\left(H^+,t^o\right)CH_3COOC_2H_5+H_2O\\ n_{CH_3COOH}=\dfrac{50}{200}.0,2=0,05\left(mol\right)\\ n_{C_2H_5OH}=\dfrac{23}{46}=0,5\left(mol\right)\\ Vì:\dfrac{0,5}{1}>\dfrac{0,05}{1}\Rightarrow Rượu.dư\\ \Rightarrow n_{este\left(LT\right)}=n_{axit}=0,05\left(mol\right)\\ \Rightarrow n_{este\left(TT\right)}=80\%.0,05=0,04\left(mol\right)\\ m_{CH_3COOC_2H_5}=88.0,04=3,52\left(g\right)\)