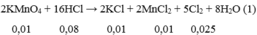Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Mg}=0,3\left(mol\right)\)
\(n_{HCl}=0,3\left(mol\right)\)
\(PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\)
...............0,15......0,3..........0,15.....0,15......
- Thấy sau phản ứng HCl phản ứng hết, Mg còn dư ( dư 0,15 mol )
\(\Rightarrow\left\{{}\begin{matrix}m_M=m_{MgCl_2}=14,25\left(g\right)\\V=V_{H_2}=3,36\left(l\right)\end{matrix}\right.\)
PT: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
Ta có: \(n_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\)
\(n_{HCl}=0,2.1,5=0,3\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,3}{1}>\dfrac{0,3}{2}\), ta được Mg dư.
Theo PT: \(n_{MgCl_2}=n_{H_2}=\dfrac{1}{2}n_{HCl}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{MgCl_2}=0,15.95=14,25\left(g\right)\\V_{H_2}=0,15.22,4=3,36\left(l\right)\end{matrix}\right.\)
Bạn tham khảo nhé!

\(n_{Mg}=\dfrac{6}{24}=0,25\left(mol\right)\\ PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
0,25 ---> 0,5 ---> 0,25 ---> 0,25
\(V_{H_2}=0,25.22,4=5,6\left(l\right)\\ m_{MgCl_2}=0,25.95=23,75\left(g\right)\\ m_{HCl}=0,5.36,5=18,25\left(g\right)\\ m_{ddHCl}=\dfrac{18,25}{18,25\%}=100\left(g\right)\\ m_{H_2}=0,25.2=0,5\left(g\right)\\ m_{dd}=100+6-0,5=105,5\left(g\right)\\ C\%_{MgCl_2}=\dfrac{23,75}{105,5}=22,51\%\)

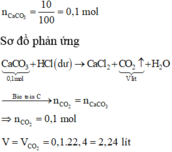
\(a,Fe+2HCl\rightarrow FeCl_2+H_2\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\Rightarrow n_{Fe}=n_{H_2}=0,1\left(mol\right)\\ \Rightarrow\%m_{Fe}=\dfrac{0,1.56}{13,6}.100\%\approx41,176\%\\ \Rightarrow\%m_{CuO}\approx58,824\%\\ b,n_{CuO}=\dfrac{13,6-0,1.56}{80}=0,1\left(mol\right)\\ n_{HCl\left(p.ứ\right)}=2.\left(n_{Fe}+n_{CuO}\right)=2.\left(0,1+0,1\right)=0,4\left(mol\right)\\ \Rightarrow V_{ddHCl}=\dfrac{0,4}{2}=0,2\left(l\right)\)

- Vì hỗn hợp rắn qua dd HCl có thấy H2 nên hh rắn chắc chắn có Al dư
\(PTHH:\left(a\right)4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ \left(b\right)Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\\ \left(c\right)2Al_{dư}+6HCl\rightarrow2AlCl_3+3H_2\\ n_{H_2\left(c\right)}=\dfrac{1,68}{22,4}=0,075\left(mol\right)\\ n_{AlCl_3\left(tổng\right)}=\dfrac{13,35}{133,5}=0,1\left(mol\right)\\ n_{AlCl_3\left(c\right)}=n_{Al\left(dư\right)}=\dfrac{2}{3}.n_{H_2\left(c\right)}=\dfrac{2.0,075}{3}=0,05\left(mol\right)\\ \Rightarrow n_{AlCl_3\left(b\right)}=0,1-0,05=0,05\left(mol\right)\\ \Rightarrow n_{HCl}=\dfrac{6}{2}.n_{AlCl_3\left(tổng\right)}=\dfrac{6}{2}.0,1=0,3\left(mol\right)\\ \Rightarrow V=V_{ddHCl}=\dfrac{0,3}{1}=0,3\left(l\right)\\ n_{Al_2O_3}=\dfrac{n_{AlCl_3\left(b\right)}}{2}=\dfrac{0,05}{2}=0,025\left(mol\right)\)
\(\Rightarrow n_{Al\left(a\right)}=2.n_{Al_2O_3}=2.0,025=0,05\left(mol\right)\\ \Rightarrow n_{Al\left(tổng\right)}=n_{Al\left(a\right)}+n_{Al\left(c\right)}=0,05+0,05=0,1\left(mol\right)\\ \Rightarrow m=m_{Al\left(tổng\right)}=0,1.27=2,7\left(g\right)\\ n_{O_2}=\dfrac{3}{2}.n_{Al_2O_3}=\dfrac{3}{2}.0,025=0,0375\left(mol\right)\\ \Rightarrow V=V_{O_2\left(đktc\right)}=0,0375.22,4=0,84\left(l\right)\)

`1)`
`n_{Al}={2,7}/{27}=0,1(mol)`
`2Al+3H_2SO_4->Al_2(SO_4)_3+3H_2`
`0,1->0,15->0,05->0,15(mol)`
`V_{dd\ H_2SO_4}={0,15}/1=0,15(l)=150(ml)`
`->V=150`
`V'=V_{H_2}=0,15.22,4=3,36(l)`
`C_{M\ X}=C_{M\ Al_2(SO_4)_3}={0,05}/{0,15}=1/3M`
`2)`
`n_{Fe}={2,8}/{56}=0,05(mol)`
`Fe+2HCl->FeCl_2+H_2`
`0,05->0,1->0,05->0,05(mol)`
`V_{dd\ HCl}={0,1}/1=0,1(l)=100(ml)`
`->V=100`
`V_{H_2}=0,05.22,4=1,12(l)`
`C_{M\ FeCl_2}={0,05}/{0,1}=0,5M`