Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(Mg+2HCl\rightarrow MgCl_2+H_2\)
b, \(m_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\)
Theo PT: \(n_{MgCl_2}=n_{Mg}=0,3\left(mol\right)\Rightarrow m_{MgCl_2}=0,3.95=28,5\left(g\right)\)
c, \(n_{HCl}=2n_{Mg}=0,6\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,6}{0,2}=3\left(M\right)\)

nCO2=0,4(mol)
a) PTHH: 2 NaOH + CO2 -> Na2CO3 + H2O
0,8_________0,4________0,4(mol)
=> mNaOH=0,8.40=32(g)
=>C%ddNaOH=(32/200).100=16%
b) mddNa2CO3=mddNaOH+mCO2=200+0,4.44=217,6(g)
mNa2CO3=106.0,4=42,4(g)
=>C%ddNa2CO3=(42,4/217,6).100=19,485%
Chúc em học tốt!
nCO2=8,96/22,4=0,4mol
a/ CO2+2NaOH→Na2CO3+H2O
0,4 0,8 0,4 0,4
mNaOH=0,8.40=32g
C%ddNaOH=mct/mdd.100%=32/200.100%=16%
b/mCO2=0,4.44=17,6g
Theo định luật bảo toàn khối lượng:
mCO2+mNaOH=mNa2CO3
17,6g+200g=217,6g
mNa2CO3=0,4.106=42,4g
C%ddNa2CO3=mct/mdd.100%=42,4/217,6.100=19,4852g

a) 2Al + 6HCl --> 2AlCl3 + 3H2
b) \(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\); nHCl = 0,5.2 = 1 (mol)
Xét tỉ lệ: \(\dfrac{0,1}{2}< \dfrac{1}{6}\) => Al hết, HCl dư
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
0,1->0,3---->0,1---->0,15
=> VH2 = 0,15.22,4 = 3,36 (l)
c) \(\left\{{}\begin{matrix}C_{M\left(AlCl_3\right)}=\dfrac{0,1}{0,5}=0,2M\\C_{M\left(HCl.dư\right)}=\dfrac{1-0,3}{0,5}=1,4M\end{matrix}\right.\)

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1 ( mol )
\(m_{Zn}=0,1.65=6,5g\)
\(C_{M_{HCl}}=\dfrac{0,2}{0,25}=0,8M\)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\
pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1
\(m_{Zn}=0,1.65=6,5g\\
C_{M\left(HCl\right)}=\dfrac{0,2}{0,25}=0,8M\)
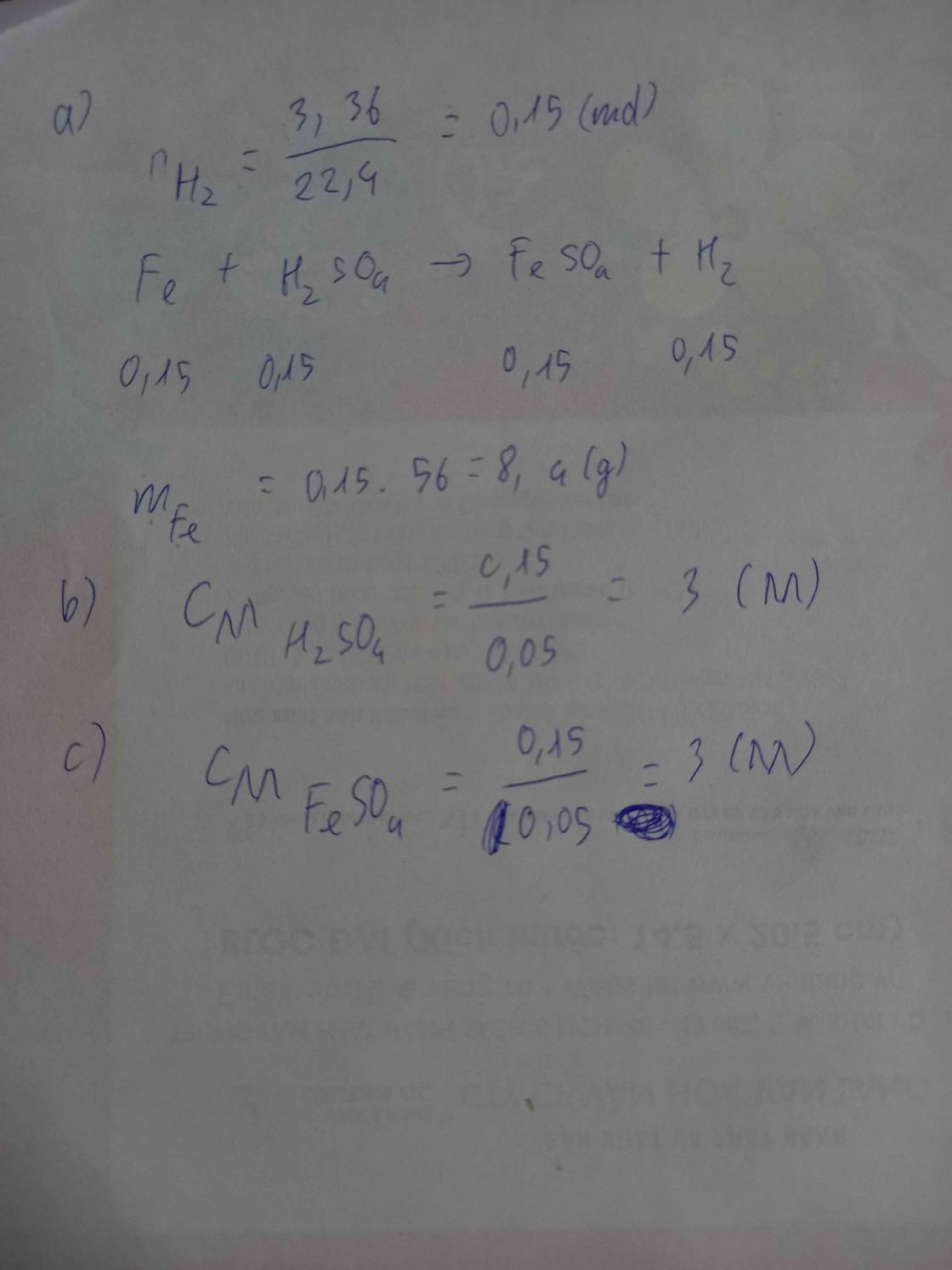
\(n_{MgCO_3}=\dfrac{16,8}{84}=0,2mol\\ a.MgCO_3+H_2SO_4->MgSO_4+H_2O+CO_2\\ 2NaOH+H_2SO_{\text{4 }}->Na_2SO_4+2H_2O\\ b.n_{H_2SO_4dư}=\dfrac{1}{2}n_{NaOH}=\dfrac{1}{2}.80.0,1:40=0,1mol\\ n_{H_2SO_4\left(MgCO_3\right)}=0,2mol\\ c.C\%=\dfrac{98.0,3}{200}.100\%=14,7\%\\ V=0,2.22,4=4,48L\\ d.m_{ddsau}=200+16,8-44.0,2+80=288g\\ C\%_{Na_2SO_4}=\dfrac{40.0,1}{288}.100\%=1,39\%\\ C\%_{MgSO_4}=\dfrac{120.0,2}{288}.100\%=8,33\%\)
\(n_{MgCO_3}=\dfrac{16,8}{84}=0,2\left(mol\right)\)
\(n_{NaOH}=\dfrac{80}{40}=2\left(mol\right)\)
PTHH :
\(MgCO_3+H_2SO_4\rightarrow MgSO_4+H_2O+CO_2\uparrow\)
0,2 0,2 0,2
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
2 1 1
Vậy có 0,2 mol H2SO4 phản ứng với MgCO3
có 1 mol H2SO4 phản ứng với NaOH
\(m_{H_2SO_4}=1,2.98=117,6\left(g\right)\)
\(c,C\%_{H_2SO_4}=\dfrac{117,6}{200}.100\%=58,8\%\)
\(V_{CO_2}=0,2.22,4=4,48\left(l\right)\)
\(d,m_{Na_2SO_4}=1.142=142\left(g\right)\)
\(m_{ddNaOH}=\dfrac{80.100}{10}=800\left(g\right)\)
\(m_{ddH_2SO_4dư}=1.98:58,8\%\approx166,67\left(g\right)\)
\(m_{ddNa_2SO_4}=800+166,67=966,67\left(g\right)\)
\(C\%_{Na_2SO_4}=\dfrac{142}{966,67}.100\%\approx14,69\%\)