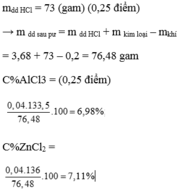
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
b) \(n_{CaCO_3}=\dfrac{10}{100}=0,1\left(mol\right)\)
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
_0,1---->0,2------->0,1----->0,1
=> mCaCl2 = 0,1.111 = 11,1 (g)
=> VCO2 = 0,1.22,4 = 2,24 (l)
c) \(a=C_{M\left(HCl\right)}=\dfrac{0,2}{0,4}=0,5M\)
d) \(C_{M\left(CaCl_2\right)}=\dfrac{0,1}{0,4}=0,25M\)

a. PTHH: \(MnO_2+4HCl\rightarrow MnCl_2+2H_2O+Cl_2\\ 0,9mol:3,6mol\rightarrow0,9mol:1,8mol:0,9mol\)
\(m_{MnO_2}=\dfrac{78,3}{87}=0,9\left(mol\right)\)
\(m_{CtHCl}=3,6.36,5=131,4\left(g\right)\)
\(C\%=\dfrac{m_{Ct}}{m_{Dd}}.100\%\)
\(\Leftrightarrow20\%=\dfrac{131,4}{m_{Dd}}.100\%\)
\(\Leftrightarrow m_{DdHCl}=657\left(g\right)\)
\(V_{Cl_2}=22,4.0,9=20,16\left(l\right)\)
b. \(m_{CtMnCl_2}=0,9.126=113,4\left(g\right)\)
\(m_{dd}=78,3+657-\left(0,9.71\right)=671,4\left(g\right)\)
\(C\%=\dfrac{113,4}{671,4}.100\%=16,89\%\)
c. \(Cl_2+2NaOH\rightarrow H_2O+NaCl+NaClO\\ 0,9mol:1,8mol\rightarrow0,9mol:0,9mol:0,9mol\)
\(CM_{NaOH}=\dfrac{1,8}{0,25}=7,2\)
\(CM_{NaClO}=\dfrac{0,9}{0,25}=3,6\)

Fe+2HCl->FeCl2+H2
x---2x-----------x
Mg+2HCl->MgCl2+H2
y------2y-----------y
Ta có :
\(\left\{{}\begin{matrix}56x+24y=24\\x+y=\dfrac{13,44}{22,4}\end{matrix}\right.\)
=>x=0,3 mol, y=0,3 mol
=>%m Fe=\(\dfrac{0,3.56}{24}.100\)=70%
=>%m Mg=100-70=30%
=>VHCl=\(\dfrac{0,3.2+0,3.2}{2}\)=0,6l=600ml
b)
XCl2+2AgNO3->2AgCl+X(NO3)2
0,6--------------------1,2mol
=>m AgCl=1,2.143,5=172,2g