Tính khối lượng của hỗn hợp gồm 2,24 lít SO2 và 1,12 lít O2 (ĐKTC)
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_{SO_2}=\frac{V_{SO_2}}{22,4}=\frac{2,24}{22,4}=0,1\left(mol\right)\)
\(=>m_{SO_2}=n_{SO_2}.M_{SO_2}=0,1.64=6,4\left(g\right)\)
\(n_{O_2}=\frac{V_{O_2}}{22,4}=\frac{3,36}{22,4}=0,15\left(mol\right)\)
\(=>m_{O_2}=n_{O_2}.M_{O_2}=0,15.32=4,8\left(g\right)\)

\(a.m_{CO_2}=3.44=132\left(g\right)\\ m_{CO}=2.28=56\left(g\right)\\b. m_{SO_2}=0,1.64=64\left(g\right)\\ m_{O_2}=0,05.32=16\left(g\right)\)

a, khối lượng của 2,5 mol CuO là:
\(m=n.M=2,5.80=200\left(g\right)\)
b, số mol của 4,48 lít khí CO2 (đktc) là:
\(n=\dfrac{V}{22,4}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)

N phân tử = 1 mol phân tử
\(\Rightarrow n_{O2}=1mol;n_{N_2}=2mol;n_{CO_2}=1,5mol\)
\(\Rightarrow m_{hh}=1.32+2.28+1,5.44=154g\)
b. \(m_{hh}=0,1.56+0,2.64+0,3.65+0,25.27=44,65g\)
c. \(n_{O_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(n_{H_2}=\dfrac{1,12}{22,4}=0,05mol\)
\(n_{HCl}=\dfrac{6,72}{22,4}=0,3mol\)
\(n_{CO_2}=\dfrac{0,56}{22,4}=0,025mol\)
\(\Rightarrow m_{hh}=0,1.32+0,05.2+0,3.36,5+0,025.44=15,35g\)

Trong A :
\(n_{CO_2}=n_X=a\left(mol\right)\)
Trong B:
\(n_{N_2}=2b\left(mol\right),n_{CO_2}=3b\left(mol\right)\)
\(n_A=2a=0.1\left(mol\right)\Rightarrow a=0.05\)
\(n_B=5b=0.05\left(mol\right)\Rightarrow b=0.01\)
\(m=0.05\cdot44+0.05\cdot X+0.02\cdot28+0.03\cdot44=4.18\left(g\right)\)
\(\Rightarrow X=2\)
\(X:H_2\)

\(n_{O2}=\dfrac{16,8}{22,4}=0,75\left(mol\right)\)
\(m_{O2}=0,75.32=24\left(g\right)\)
⇒ \(M_{O2}=\dfrac{24}{0,75}=32\)(g/mol)
\(n_{SO2}=\dfrac{10,08}{22,4}=0,45\left(mol\right)\)
\(m_{SO2}=0,45.64=28,8\left(g\right)\)
⇒ \(M_{SO2}=\dfrac{28,8}{0,45}=64\)(g/mol)
\(n_{Cl2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\)
\(m_{Cl2}=0,35.71=24,85\left(g\right)\)
⇒ \(M_{Cl2}=\dfrac{24,85}{0,35}=71\)(g/mol)
Chúc bạn học tốt

\(1,\\ a,m_{hh}=3.44+2.28=188(g)\\ b,m_{hh}=\dfrac{2,24}{22,4}.64+\dfrac{1,12}{22,4}.32=8(g)\\ 2,\\ a,V_{hh}=(\dfrac{4,4}{44}+\dfrac{0,4}{2}).22,4=6,72(l)\\ b,V_{hh}=(\dfrac{6.10^{23}}{6.10^{23}}+\dfrac{3.10^{23}}{6.10^{23}}).22,4=33,6(l)\)
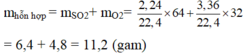
\(n_{SO_2\left(dktc\right)}=\dfrac{V}{22,4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ m_{SO_2}=n\cdot M=0,1\cdot\left(32+16\cdot2\right)=6,4\left(g\right)\)
\(n_{O_2\left(dktc\right)}=\dfrac{V}{22,4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\\ m_{O_2}=n\cdot M=0,05\cdot32=1,6\left(g\right)\)
\(=>m_{hh}=1,6+6,4=8\left(g\right)\)
\(n_{SO_2}=\dfrac{V_{\left(\text{đ}ktc\right)}}{22,4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ \Rightarrow m_{SO_2}=n.M=0,1.64=6,4\left(g\right)\\ n_{O_2}=\dfrac{V_{\left(\text{đ}ktc\right)}}{22,4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\\ \Rightarrow m_{O_2}=n.M=0,05.32=1,6\left(g\right)\\ \Rightarrow m_{hh}=m_{SO_2}+m_{O_2}=6,4+1,6=8\left(g\right)\)