Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, khối lượng của 2,5 mol CuO là:
\(m=n.M=2,5.80=200\left(g\right)\)
b, số mol của 4,48 lít khí CO2 (đktc) là:
\(n=\dfrac{V}{22,4}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)

mMg = 0,5.24 = 12 gam
VSO2 = n.22,4 = 0,25.22,4 = 5,6 lít
nN2 = \(\dfrac{16,8}{22,4}\)= 0,75 mol , nO2 = \(\dfrac{5,6}{22,4}\)= 0,25 mol
=> m(N2 + O2 ) = 0,75.28 + 0,25.32 = 29 gam

\(\left\{{}\begin{matrix}n_{Cl_2}+n_{O_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\\dfrac{n_{Cl_2}}{n_{O_2}}=\dfrac{1}{3}\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}n_{Cl_2}=0,1\left(mol\right)\\n_{O_2}=0,3\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}m_{Cl_2}=0,1.71=7,1\left(g\right)\\m_{O_2}=0,3.32=9,6\left(g\right)\end{matrix}\right.\)
=> mhh = 7,1 + 9,6 = 16,7(g)
Đặt $n_{Cl_2}=x(mol)\Rightarrow n_{O_2}=3x(mol)$
Mà $n_{hh}=n_{Cl_2}+n_{O_2}=\dfrac{8,96}{22,4}=0,4$
$\Rightarrow x+3x=0,4\Rightarrow x=0,1$
$\Rightarrow m_{Cl_2}=0,1.71=7,1(g);m_{O_2}=3.0,1.32=9,6(g)$
$\Rightarrow m_{hh}=7,1+9,6=16,7(g)$

\(n_{Cl_2}=a\left(mol\right)\)
\(n_{Mg}=b\left(mol\right)\)
\(n_X=a+b=\dfrac{7.84}{22.4}=0.35\left(mol\right)\left(1\right)\)
Bảo toàn khối lượng :
\(m_{Cl_2}+m_{O_2}=30.1-11.1=19\left(g\right)\)
\(\Leftrightarrow71a+32b=19\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.2,b=0.15\)
\(Đặt:\)
\(n_{Mg}=x\left(mol\right),n_{Al}=y\left(mol\right)\)
\(m_Y=24x+27y=11.1\left(g\right)\left(3\right)\)
Bảo toàn e :
\(2x+3y=0.2\cdot2+0.15\cdot4=1\left(4\right)\)
\(\left(3\right),\left(4\right):x=0.35,y=0.1\)
\(\%Mg=\dfrac{0.35\cdot24}{11.1}\cdot100\%=75.67\%\)
\(\%Al=24.33\%\)

\(n_{SO_2}=\frac{V_{SO_2}}{22,4}=\frac{2,24}{22,4}=0,1\left(mol\right)\)
\(=>m_{SO_2}=n_{SO_2}.M_{SO_2}=0,1.64=6,4\left(g\right)\)
\(n_{O_2}=\frac{V_{O_2}}{22,4}=\frac{3,36}{22,4}=0,15\left(mol\right)\)
\(=>m_{O_2}=n_{O_2}.M_{O_2}=0,15.32=4,8\left(g\right)\)

a, VO\(_2\) = 0,15 . 22,4 = 3,36 lít
b, V\(CO_2\) = \((\dfrac{48}{44}).22,4\approx24,43\) ( lít )
c, \(V_{SO_2}=\left(\dfrac{16}{64}\right).22,4=5,6\) ( lít )
\(V_{H_2}=\left(\dfrac{18.10^{23}}{6.10^{23}}\right).22,4=67,2\) ( lít )
=> \(V_{hh}=5,6+67,2=72,8\) ( lít )
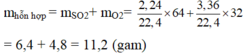
\(n_{O2}=\dfrac{16,8}{22,4}=0,75\left(mol\right)\)
\(m_{O2}=0,75.32=24\left(g\right)\)
⇒ \(M_{O2}=\dfrac{24}{0,75}=32\)(g/mol)
\(n_{SO2}=\dfrac{10,08}{22,4}=0,45\left(mol\right)\)
\(m_{SO2}=0,45.64=28,8\left(g\right)\)
⇒ \(M_{SO2}=\dfrac{28,8}{0,45}=64\)(g/mol)
\(n_{Cl2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\)
\(m_{Cl2}=0,35.71=24,85\left(g\right)\)
⇒ \(M_{Cl2}=\dfrac{24,85}{0,35}=71\)(g/mol)
Chúc bạn học tốt