. Cho nhôm tác dụng với dung dịch H,SO, loãng sinh ra 4,48 lít khí H, ở đktc. a) Viết phương trình phản ứng xảy ra. b) Tính khối lượng nhôm đã phản ứng.
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\ a,2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ b,n_{Al}=\dfrac{2}{3}.n_{H_2}=\dfrac{2}{3}.0,2=\dfrac{2}{15}\left(mol\right)\\ \Rightarrow m_{Al}=\dfrac{2}{15}.27=3,6\left(g\right)\)

\(\left(a\right)2Al+3H_2O\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ \left(b\right)n_{H_2}=\dfrac{13,44}{22,4}=0,6mol\\ n_{Al}=\dfrac{0,6.2}{3}=0,4mol\\ m_{Al}=0,4.27=10,8g\\ \left(c\right)n_{O_2}=\dfrac{4,8}{32}=0,15mol\\ 4Al+3O_2\underrightarrow{t^0}2Al_2O_3\\ \Rightarrow\dfrac{0,4}{4}>\dfrac{0,15}{3}\Rightarrow Al.dư\\ n_{Al_2O_3}=\dfrac{0,15.2}{3}=0,1mol\\ m_{oxit}=m_{Al_2O_3}=0,1.102=10,2g\)
a: \(2Al+3H_2SO_4\rightarrow1Al_2\left(SO_4\right)_3+3H_2\uparrow\)
0,4 0,6 0,2 0,6
b: \(n_{H_2}=\dfrac{13.44}{22.4}=0.6\left(mol\right)\)
=>\(n_{Al}=0.4\left(mol\right)\)
\(m_{Al}=0.4\cdot27=10.8\left(g\right)\)
c: \(4Al+3O_2\rightarrow2Al_2O_3\)
0,4 0,2
\(m_{Al_2O_3}=0.2\left(27\cdot2+16\cdot3\right)=0.2\cdot102=20.4\left(g\right)\)

`a)PTHH:`
`2Al + 6HCl -> 2AlCl_3 + 3H_2`
`0,2` `0,6` `0,3` `(mol)`
`n_[Al]=[5,4]/27=0,2(mol)`
`b)V_[H_2]=0,3.22,4=6,72(l)`
`c)m_[dd HCl]=[0,6.36,5]/10 . 100 =219(g)`

HD:
a,
2AL+3H2SO4=>AL2(SO4)3+3H2
b,
Ta có: nAL=10.8/27=0.4(mol)
theo phương trình ta có: nH2=3/2nAL=0.6(mol)
=> VCO2=0.6*22.4=13.44(lít)
c,
Ta có: nH2=11.2/22.4=0.5(mol)
theo phương trình ta có: nH2SO4=nH2=0.5(mol)
=>mH2SO4=0.5*98=49(g)

2Al+3H2SO4->Al2(SO4)3+3H2
0,1----------------------0,075----0,15
n H2=0,15 mol
=>mAl=0,1.27=2,7g
=>m Al2(SO4)3=0,075.342=25,65g
a) PTHH: \(2Al+3H_2SO_2\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
b) \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(n_{Al}=\dfrac{2}{3}.0,15=0,1\left(mol\right)\)
\(m_{Al}=0,1.27=2,7\left(g\right)\)
c) \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}.0,1=0,05\left(mol\right)\)
\(m_{Al_2\left(SO_4\right)_3}=0,05.342=17,1\left(g\right)\)

\(n_{HCl}=\dfrac{25}{36,5}=\dfrac{50}{73}mol\)
2Al + 6HCl \(\rightarrow\) 2AlCl3 + 3H2
\(\Rightarrow n_{Al}=\dfrac{\dfrac{50}{73}.2}{6}=\dfrac{50}{219}mol\\ m_{Al}=\dfrac{50}{219}.27=\dfrac{450}{73}g\)
\(n_{H_2}=\dfrac{\dfrac{50}{73}.3}{6}=\dfrac{25}{73}mol\\ V_{H_2}=\dfrac{25}{73}.22,4=\dfrac{560}{73}l\)
a: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
b: \(n_{HCl}=\dfrac{25}{36.5}=\dfrac{50}{73}\left(mol\right)\)
\(\Leftrightarrow n_{AlCl_3}=\dfrac{150}{73}\left(mol\right)=n_{Al}\)
\(m_{Al}=\dfrac{150}{73}\cdot27=\dfrac{4050}{73}\left(g\right)\)

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
a) Pt : \(Mg+H_2SO_4\rightarrow MgSO_4+H_2|\)
1 1 1 1
0,1 0,1
b) \(n_{H2}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,1.22,4=2,24\left(l\right)\)
Chúc bạn học tốt
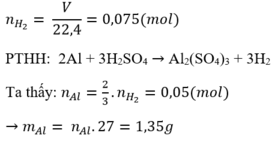
\(n_{H2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
a) Pt : \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2|\)
2 3 1 3
\(\dfrac{2}{15}\) 0,2
b) \(n_{Al}=\dfrac{0,2.2}{3}=\dfrac{2}{15}\left(mol\right)\)
⇒ \(m_{Al}=\dfrac{2}{15}.27=3,6\left(g\right)\)
Chúc bạn học tốt