Btvn: cho các chất:Cu,CuO,Cu(oh)2 ,cucl2,cu(no3)2 và cuso4 Sắp xếp thành dãy chuyển hoá và viết PTHH xảy ra.
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\\ CuSO_4+Ba\left(NO_3\right)_2\rightarrow BaSO_4+Cu\left(NO_3\right)_2\\ Cu\left(NO_3\right)_2+2KOH\rightarrow Cu\left(OH\right)_2+2KNO_3\\ Cu\left(OH\right)_2\rightarrow\left(t^o\right)CuO+H_2O\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ CuCl_2\rightarrow\left(t^o\right)Cu+Cl_2\)

a)
(1) \(Cu+2H2SO4\left(đ\right)-^{t0}->CuSO4+SO2\uparrow+2H2O\)
\(\left(2\right)CuSO4+BaCl2->CuCl2+BaSO4\downarrow\)
(3) \(CuCl2+2AgNO3->Cu\left(NO3\right)2+2AgCl\downarrow\)
(4) \(Cu\left(NO3\right)2+2NaOH->Cu\left(OH\right)2\downarrow+2NaNO3\)
(5) \(Cu\left(OH\right)2-^{t0}->CuO+H2O\)
(6) \(CuO+2HCl->CuCl2+H2O\)
(7) \(CuCl2-^{t0}->Cu+Cl2\uparrow\)
(8) \(Cu+Cl2-^{t0}->CuCl2\)
(9) \(CuCl2+2NaOh->Cu\left(OH\right)2\downarrow+2NaCl\)
b)
\(4Na+O2-^{t0}->2Na2O\)
\(Na2O+H2O->2NaOH\)
\(2NaOH+CO2->Na2CO3+H2O\)
\(Na2CO3+H2SO4->Na2SO4+CO2\uparrow+H2O\)
\(Na2SO4+BaCl2->2NaCl+BaSO4\downarrow\)
\(2NaCl+2H2O-\xrightarrow[có-màng-ngăn]{đpdd}2NaOH+Cl2\uparrow+H2\uparrow\)
\(3NaOH+H3PO4->Na3PO4+3H2O\)

\(Cu\overset{\left(1\right)}{-->}CuO\overset{\left(2\right)}{-->}CuSO_4\overset{\left(3\right)}{-->}CuCl_2\overset{\left(4\right)}{-->}Cu\left(NO_3\right)_2\overset{\left(5\right)}{-->}Cu\left(OH\right)_2\overset{\left(6\right)}{-->}CuO\overset{\left(7\right)}{-->}Cu\)
\(\left(1\right)2Cu+O_2\overset{t^o}{--->}2CuO\)
\(\left(2\right)CuO+H_2SO_4--->CuSO_4+H_2O\)
\(\left(3\right)CuSO_4+BaCl_2--->BaSO_4\downarrow+CuCl_2\)
\(\left(4\right)CuCl_2+2AgNO_3--->2AgCl\downarrow+Cu\left(NO_3\right)_2\)
\(\left(5\right)Cu\left(NO_3\right)_2+2NaOH--->Cu\left(OH\right)_2\downarrow+2NaNO_3\)
\(\left(6\right)Cu\left(OH\right)_2\overset{t^o}{--->}CuO+H_2O\)
\(\left(7\right)CuO+H_2\overset{t^o}{--->}Cu+H_2O\)
\(2Cu+O_2\underrightarrow{t^o}2Cuo\)
\(CuO+H_2SO_4->CuSO_4+H_2O\)
\(CuSO_4+BaCl_2->BaSO_4\downarrow+CuCl_2\)
\(CuCl_2+2AgNO_3->Cu\left(NO_3\right)_2+2AgCl\downarrow\)
\(Cu\left(NO_3\right)_2+2NaOH->Cu\left(OH\right)_2\downarrow+2NaNO_3\)
\(Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)
\(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)

Tham khảo
(1) 2Cu + O2 ---> 2CuO
(2) CuO + H2SO4 ---> CuSO4 + H2
(3) CuSO4 + 2NaOH ---> Cu(OH)2 + Na2SO4
(4) Cu(OH)2 + 2HCl ---> CuCl2 + 2H2O
(5) CuCl2 + 2AgNO3 ----> Cu(NO3)2 + 2AgCl

\(2Cu+O_2\underrightarrow{t^o}2CuO\)
\(CuO+2HCl->CuCl_2+H_2O\)
\(CuCl_2+2NaOH->Cu\left(OH\right)_2\downarrow+2NaCl\)
\(Cu\left(OH\right)_2+H_2SO_4->CuSO_4+2H_2O\)
\(CuSO_4+Ba\left(NO_3\right)_2->Cu\left(NO_3\right)_2+BaSO_4\downarrow\)

\(Cu+Cl_2\xrightarrow[]{t^0}CuCl_2\\ CuCl_2+2AgNO_3\rightarrow Cu\left(NO_3\right)_2+2AgCl\\ Cu\left(NO_3\right)_2+2KOH\rightarrow Cu\left(OH\right)_2+2KNO_3\\ Cu\left(OH\right)_2\xrightarrow[]{t^0}CuO+H_2O\\ CuO+H_2SO_4\rightarrow CuSO_4+H_2O\\ CuSO_4+Mg\rightarrow MgSO_4+Cu\)
Cu+Cl2➝CuCl2
CuCl2+2AgNO3➝Cu(NO3)2+2AgCl↓
Cu(NO3)2+2NaOH➝ Cu(OH)2↓+2NaNO3
Cu(OH)2➝CuO+H2O
CuO+H2SO4➝CuSO4+H2O
CuSO4+Mg(OH)2➝Cu(OH)2↓+MgSO4
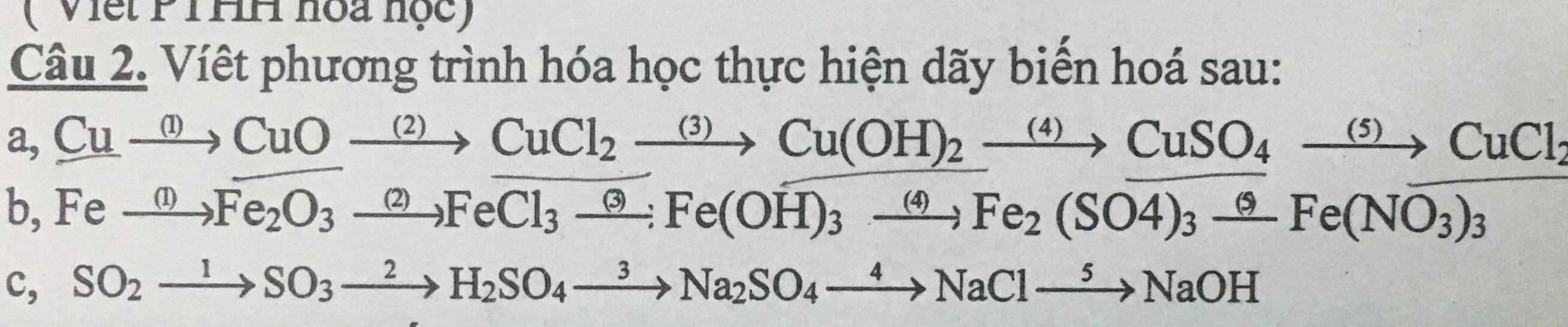
$Cu \to CuO \to CuSO_4 \to CuCl_2 \to Cu(NO_3)_2 \to Cu(OH)_2$
$2Cu + O_2 \xrightarrow{t^o} 2CuO$
$CuO + H_2SO_4 \to CuSO_4 + H_2O$
$CuSO_4 + BaCl_2 \to BaSO_4 + CuCl_2$
$CuCl_2 + 2AgNO_3 \to Cu(NO_3)_2 + 2AgCl$
$Cu(NO_3)_2 + 2KOH \to Cu(OH)_2 + 2KNO_3$
CuSO4 ------> CuCl2--------> Cu(NO3)2 -------> Cu(OH)2 ------> CuO-----> Cu
\(CuSO_4+BaCl_2\rightarrow BaSO_4+CuCl_2\)
\(CuCl_2+2AgNO_3\rightarrow Cu\left(NO_3\right)_2+2AgCl\)
\(Cu\left(NO_3\right)_2+2NaOH\rightarrow Cu\left(OH\right)_2+2NaNO_3\)
\(Cu\left(OH\right)_2-^{t^o}\rightarrow CuO+H_2O\)
\(CuO+H_2-^{t^o}\rightarrow Cu+H_2O\)