Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{KMnO_4}=\dfrac{m_{KMnO_4}}{M_{KMnO_4}}=\dfrac{47,4}{158}=0,3mol\)
\(n_{KMnO_4}=\dfrac{0,3}{80\%}=0,375mol\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
2 16 2 2 5 8 ( mol )
0,375 > 2,5 ( mol )
0,375 0,9375 ( mol )
\(V_{Cl_2}=n_{Cl_2}.22,4=0,9375.22,4=21l\)
\(n_{KMnO_4\left(bd\right)}=\dfrac{47,4}{158}=0,3\left(mol\right)\) => \(n_{KMnO_4\left(pư\right)}=\dfrac{0,3.80}{100}=0,24\left(mol\right)\)
PTHH: 2KMnO4 + 16HCl --> 2KCl + 2MnCl2 + 5Cl2 + 8H2O
0,24------------------------------------->0,6
=> \(V=0,6.22,4=13,44\left(l\right)\)

\(n_{MnO_2}=\dfrac{17,4}{87}=0,2\left(mol\right)\\ PTHH:MnO_2+4HCl_{đặc,nóng}\rightarrow MnCl_2+Cl_2+2H_2O\\ n_{Cl_2\left(TT\right)}=\dfrac{3,584}{22,4}=0,16\left(mol\right)\\ n_{Cl_2\left(LT\right)}=n_{MnO_2}=0,2\left(mol\right)\\ \Rightarrow H=\dfrac{n_{Cl_2\left(TT\right)}}{n_{Cl_2\left(LT\right)}}.100\%=\dfrac{0,16}{0,2}.100=80\%\)

MnO\(_2\)+4HCl\(\rightarrow\)MnCl\(_2\)+Cl\(_2\)+2H\(_2O\)
0,45 0,45 (mol)
n\(_{MnO_2}\)=\(\dfrac{39,15}{87}\)=0,45(mol)
2Fe + 3Cl\(_2\)\(\rightarrow\)2FeCl\(_3\)
0,3 0,45 0,3 (mol)
m\(_{FeCl_3}\)=0,3.162,5=48,75(g)
vì hiệu suất phản ứng là 86% nên:
m\(_{FeCl_3}\)=\(\dfrac{86.48,75}{100}\)=41,925(g)
2/
Mg+Cl\(_2\)\(\rightarrow\)MnCl\(_2\)
0,6 0,6
n\(_{Mg}\)=\(\dfrac{14,4}{24}\)=0,6(mol)
2\(KMnO_4+16HCl\rightarrow2MnCl_2+2KCl+5Cl_2\uparrow+8H_2O\)
0,24 0,6
vì hiệu suất phản ứng bằng 80%,nên để điều chế 0,6 mol Cl\(_2\)thì cần số mol \(KMnO_4\) là:
n\(_{KMnO_4}\)=\(\dfrac{0,24.100}{80}\)=0,3(mol)
m\(KMnO_4\)=0,3.158=47,4(g)

Ta có nCl2 = 8,96/22,4 = 0,4 mol
PTHH :
2KMnO4 + 16HCl - > 2KCl + 2MnCl2 + 5Cl2 + 8H2O
0,16mol.........1,28mol...............................0,4mol
=> Khối lượng của KMnO4 là : mKMnO4 = 0,16.158=25,28(g)
Khối lượng dd HCl là : mddHCl = \(\frac{1,28.36,5.100}{19,2}\approx243,33\left(g\right)\)
Vì hiệu suất là 80% nên
=> \(\left\{{}\begin{matrix}mKMnO4=\frac{25,28.80}{100}=20,224\left(g\right)\\mddHCl=\frac{243,33.80}{100}=194,664\left(g\right)\end{matrix}\right.\)

8,96l Cl2 + H2 ---H=75%---> 2HCl
0,4............................................0,8
V Hcl lí thuyết : 0,8 . 22,4 = 17,92 (l)
V HCl thực tế : 17,92 . 75% = 13,44 (l)
\(n_{Cl_2}=\dfrac{8,96}{22,4}=0,25\left(mol\right)\)
PT: Cl2 + H2 → 2HCl
Mol: 0,25 0,5
\(m_{HCl\left(lt\right)}=0,5.36,5=18,25\left(g\right)\)
\(\Rightarrow m_{HCl\left(tt\right)}=75\%.18,25=13,6875\left(g\right)\)

- Theo bài ra \(\Rightarrow\left\{{}\begin{matrix}n_{KMnO_4}=0,1\\n_{KClO_3}=0,15\end{matrix}\right.\) ( mol )
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
.......0,1..........................................................0,25...........
\(KClO_3+6HCl\rightarrow KCl+3Cl_2+3H_2O\)
....0,15................................0,45....................
\(\Rightarrow n_{HCl}=0,7\left(mol\right)\)
\(6KOH+3Cl_2\rightarrow KClO_3+5KCl+3H_2O\)
Ta có : \(m=m_{KOH}+m_{Cl_2}=139,3\left(g\right)\)
Vậy ...

\(m_{NaCl\left(bd\right)}=\dfrac{1.89,5.10^6}{100}=895000\left(g\right)\)
\(m_{ddHCl}=1250.10^3.1,19=1487500\left(g\right)\)
=> \(n_{HCl}=\dfrac{1487500.37\%}{36,5}=\dfrac{550375}{36,5}\left(mol\right)\)
=> \(n_{NaCl\left(pư\right)}=\dfrac{550375}{36,5}\left(mol\right)\)
=> \(H\%=\dfrac{\dfrac{550375}{36,5}.58,5}{895000}.100\%=98,56\%\)
Một tấn muối ăn chứa 10,5% tạp chất.
\(\Rightarrow\)Nó chứa 89,5% muối nguyên chất.
\(\Rightarrow m_{NaCl}=1\cdot89,5\%=0,895tấn\)
\(m_{ddHCl}=D\cdot V=1,19\cdot1250\cdot1000=1487500g\)
\(\Rightarrow m_{HCl}=\dfrac{1487500\cdot37\%}{100\%}=550375g\)
\(\Rightarrow n_{HCl}=15078,76712mol\)
\(n_{NaCl}=\dfrac{0,895\cdot10^6}{23+35,5}=15299,1453mol\)
\(H=\dfrac{n_{HCl}}{n_{NaCl}}=\dfrac{15078,76712}{15299,1453}=98,56\%\)

Ta có PTHH : 2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 5Cl2 + 8H2O
Ta có: nKMnO4= \(\dfrac{31,6}{39+55+16.4}\)
=>nKMnO4= 0,2 (mol)
Theo PTHH nCl2 75%=\(\dfrac{5}{2}\)nKMnO4= \(\dfrac{5}{2}\).0,2= 0,5 (mol)
Biết Hiệu suất của pứ là 75%
=> nCl2=\(\dfrac{0,5.75}{100}\)=0,375 (mol)
=>VCl2=0,375.22,4=8,4 (l)

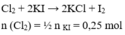
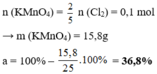
Ta có: mKMnO4 = 300.85% = 255 (kg)
\(\Rightarrow n_{KMnO_4}=\dfrac{255}{158}\left(kmol\right)\)
PT: \(2KMnO_4+16HCl_đ\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
Theo PT: \(n_{Cl_2\left(LT\right)}=\dfrac{5}{2}n_{KMnO_4}=\dfrac{1275}{316}\left(kmol\right)\)
Mà: H = 65%
\(\Rightarrow n_{Cl_2\left(TT\right)}=\dfrac{1275}{316}.65\%=\dfrac{3315}{1264}\left(kmol\right)\)
\(\Rightarrow V_{Cl_2\left(TT\right)}=\dfrac{3315}{1264}.22,4.1000\approx58746,8\left(l\right)\)