Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(\left\{{}\begin{matrix}nNaCl=x\\nNaBr=y\\nNaI=z\end{matrix}\right.\)
ta có : \(58,5x+103y+150z=5,76\left(1\right)\)
TN1:
\(Br_2+2NaI\rightarrow2NaBr+I_2\)
z ----> z
\(\Rightarrow m_{muối}=mNaBr+mNaCl=103\left(y+z\right)+58,5x=5,29\left(2\right)\)
Từ (1) và (2) => z =0,01
TN2:
\(Cl_2+2NaI\rightarrow2NaCl+I_2\)
0,01 0,01
\(Cl_2+2NaBr\rightarrow2NaCl+I_2\)
\(nNaCl=nCl^-=0,05\rightarrow mNaCl=2,925\left(g\right)\)muối khan ngoài NaCl còn muối khác.Do \(I^-\) có tính khử mạnh hơn \(Br^-\) nên NaI sẽ hết trước và sau hai phản ứng NaBr còn dư.
\(nNaBr\left(dư\right)=t\)
\(\Rightarrow nNaCl=0,05=0,01+y-t+x\)
\(m_{muối}=mNacl+mNaBr\Rightarrow mNaBr_{\left(dư\right)}=3,955-0,05.58,5=1,03\)
\(\Rightarrow t=0,01\)
\(\Rightarrow x+y=0,05\left(3\right)\)
(1) ; (3) ; => \(x=0,02;y=0,03\)
\(\Rightarrow mNaCl=1,17\left(g\right)\)

Chúc bạn học tốt !!!
\(m_{hh}=74.5a+58.5b=26.6\left(g\right)\left(1\right)\)
\(n_{AgCl}=\dfrac{57.4}{143.5}=0.4\left(mol\right)\)
\(KCl+AgNO_3\rightarrow KNO_3+AgCl\)
\(NaCl+AgNO_3\rightarrow NaNO_3+AgCl\)
\(n_{AgCl}=a+b=0.4\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):\)
\(a=b=0.2\)
\(m_{dd\left(saupư\right)}=26.6+500-57.4=469.2\left(g\right)\)
\(C\%_{KNO_3}=\dfrac{0.2\cdot101}{469.2}\cdot100\%=4.31\%\)
\(C\%_{NaNO_3}=\dfrac{0.2\cdot85}{469.2}\cdot100\%=3.62\%\)

1)
a)
$CaO + 2HCl \to CaCl_2 + H_2O$
$CaCO_3 + 2HCl \to CaCl_2 + CO_2 + H_2O$
$n_{CaCO_3} = n_{CO_2} = 0,2(mol)$
$n_{CaCl_2} = 0,3(mol)$
Suy ra:
$n_{CaO} = 0,3 - 0,2 = 0,1(mol)$
$\%m_{CaO} = \dfrac{0,1.56}{0,1.56 + 0,2.100}.100\% = 21,875\%$
$\%m_{CaCO_3} = 78,125\%$
b)
$m_{dd} = 0,1.56 + 0,2.100 + 50 - 0,2.44 = 66,8(gam)$
$C\%_{CaCl_2} = \dfrac{33,3}{66,8}.100\% = 49,85\%$
Câu 4 :
a)
Gọi $n_{Fe} = a(mol) ; n_{MgO} = b(mol)$
Suy ra: $56a + 40b = 19,2(1)$
$Fe + 2HCl \to FeCl_2 + H_2$
$MgO + 2HCl \to MgCl_2 + H_2O$
Theo PTHH : $n_{HCl} = 2a + 2b = 0,4.2 = 0,8(2)$
Từ (1)(2) suy ra a = b = 0,2
$\%m_{Fe} = \dfrac{0,2.56}{19,2}.100\% = 58,33\%$
$\%m_{MgO} = 100\% -58,33\% = 41,67\%$
b)
$n_{FeCl_2} = a = 0,2(mol)$
$n_{MgCl_2} = b = 0,2(mol)$
$m_{muối} = 0,2.127 + 0,2.95 = 44,4(gam)$

Ta có: \(\left\{{}\begin{matrix}n_{CO_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\n_{CaCl_2}=\dfrac{33,3}{111}=0,3\left(mol\right)\end{matrix}\right.\)
PTHH: \(CaCO_3+2HCl\rightarrow CaCl_2+CO_2\uparrow+H_2O\)
0,2_____0,4_____0,2____0,2_____0,2 (mol)
\(CaO+2HCl\rightarrow CaCl_2+H_2O\)
0,1_____0,2_____0,1____0,1 (mol)
Ta có: \(\left\{{}\begin{matrix}m_{CaO}=0,1\cdot56=5,6\left(g\right)\\m_{CaCO_3}=0,2\cdot100=20\left(g\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}\%m_{CaO}=\dfrac{5,6}{5,6+20}\cdot100\%=21,875\%\\\%m_{CaCO_3}=78,125\%\end{matrix}\right.\)
Mặt khác: \(m_{CO_2}=0,2\cdot44=8,8\left(g\right)\)
\(\Rightarrow m_{dd\left(sau.p/ứ\right)}=m_{CaO}+m_{CaCO_3}+m_{ddHCl}-m_{CO_2}=66,8\left(g\right)\)
\(\Rightarrow C\%_{CaCl_2}=\dfrac{33,3}{66,8}\cdot100\%\approx49,85\%\)
nCO2=\(\dfrac{4,48}{22,4}=0,2\) mol
nCaCl2=\(\dfrac{33,3}{111}=0,3\)
CaCO2 + 2HCl → CaCl2 + CO2 + H2O
0,2 ← 0,2 ← 0,2
CaO + 2HCl → CaCl2 + H2O
0,1 ← 0,1
a) % CaO=\(\dfrac{0,1.56}{0,1.56+0,2.100}.100\%=21,875\%\)
% CaCO3 =100% - 21,875%= 78,125%
b) a = mCaO+mCaCO3 =0,1.56+0,2.100=25,6g
mdd sau pư= a + mddHCl - mCO2
= 25,6 + 50 - 0,2.44=66,8g
C%CaCl2=\(\dfrac{33,3}{66,8}.100\%\simeq49,85\%\)
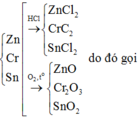
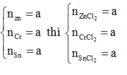

Dẫn hỗn hợp muối qua khí Cl2 dư
2NaBr + Cl2 → 2NaCl + Br2
2NaI + Cl2 → 2NaCl + I2