Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{Ba\left(OH\right)_2}=0,1.0,1=0,01\left(mol\right)=n_{Ba^{2+}}\)
\(\Rightarrow n_{OH^-}=2n_{Ba\left(OH\right)_2}=0,02\left(mol\right)\)
\(n_{NaOH}=0,1.0,1=0,01\left(mol\right)=n_{Na^+}=n_{OH^-}\)
⇒ ΣnOH- = 0,02 + 0,01 = 0,03 (mol)
\(n_{H_2SO_4}=0,4.0,0175=0,007\left(mol\right)=n_{SO_4^{2-}}\)
\(\Rightarrow n_{H^+}=2n_{H_2SO_4}=0,014\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
0,014___0,014 (mol) ⇒ nOH- dư = 0,03 - 0,014 = 0,016 (mol)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\)
0,007____0,007_____0,007 (mol) ⇒ nBa2+ dư = 0,01 - 0,007 = 0,003 (mol)
⇒ m = 0,007.233 = 1,631 (g)
\(\left[OH^-\right]=\dfrac{0,016}{0,1+0,4}=0,032\left(M\right)\)
\(\left[Ba^{2+}\right]=\dfrac{0,003}{0,1+0,4}=0,006\left(M\right)\)
\(\left[Na^+\right]=\dfrac{0,01}{0,1+0,4}=0,02\left(M\right)\)
b, pH = 14 - (-log[OH-]) ≃ 12,505
\(n_{Ba^{2+}}=0,1.0,1=0,01\left(mol\right)\)
\(n_{SO_4^{2-}}=0,4.0,0175=7.10 ^{-3}\left(mol\right)\)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\downarrow\)
\(\Rightarrow m=m_{BaSO_4}=7.10^{-3}.233=1,631\left(g\right)\)
Ta có:
\(n_{H^+}=0,4.0,0175.2=0,014\left(mol\right)\)
\(n_{OH^-}=0,1.0,1.2+0,1.0,1=0,03\left(mol\right)\)
Trong dung dịch X:
\(n_{OH^-}=0,03-0,014=0,016\left(mol\right)\)\(\Rightarrow\left[OH^-\right]=\dfrac{0,016}{0,1+0,4}=0,032\left(M\right)\)
\(n_{Ba^{2+}}=0,01-7.10^{-3}=3.10^{-3}\left(mol\right)\Rightarrow\left[Ba^{2+}\right]=\dfrac{3.10^{-3}}{0,1+0,4}=6.10^{-3}\left(M\right)\)
\(n_{Na^+}=0,1.0,1=0,01\left(mol\right)\Rightarrow\left[Na^+\right]=0,02\)
\(pOH=-lg\left(0,032\right)\approx1,5\Rightarrow pH=14-1,5=12,5\)

\(n_{Ba\left(OH\right)_2}=0,3.0,1=0,03\left(mol\right)\\ n_{HCl}=0,2.0,15=0,03\left(mol\right)\\ Ba\left(OH\right)_2+2HCl\rightarrow BaCl_2+2H_2O\\ Vì:\dfrac{n_{Ba\left(OH\right)_2\left(đề\right)}}{n_{Ba\left(OH\right)_2\left(PTHH\right)}}=\dfrac{0,03}{1}>\dfrac{n_{HCl\left(đề\right)}}{n_{HCl\left(PTHH\right)}}=\dfrac{0,03}{2}\\ \Rightarrow Ba\left(OH\right)_2dư\\ n_{Ba\left(OH\right)_2\left(p.ứ\right)}=\dfrac{n_{HCl}}{2}=\dfrac{0,03}{2}=0,015\\ n_{Ba\left(OH\right)_2\left(dư\right)}=0,03-0,015=0,015\left(mol\right)\\ \left[OH^-\right]=2.\left[Ba\left(OH\right)_2\left(dư\right)\right]=\dfrac{0,015}{0,3+0,2}=0,03\left(M\right)\\ \Rightarrow pH=14+log\left[OH^-\right]=14+log\left[0,03\right]\approx12,477\)
Nồng độ mol/lít các ion trong dd A:
\(\left[OH^-\left(dư\right)\right]=0,06\left(M\right)\left(nt\right)\\\left[Cl^-\right]=2.\left[BaCl_2\right]=2.\left(\dfrac{0,015}{0,5}\right)=0,06\left(M\right)\\ \left[Ba^{2+}\right]=0,03+ 0,03=0,06\left(M\right)\)

a, \(n_{HCl}=0,1.0,2=0,02\left(mol\right)=n_{H^+}=n_{Cl^-}\)
\(n_{H_2SO_4}=0,1.0,2=0,02\left(mol\right)=n_{SO_4^{2-}}\) \(\Rightarrow n_{H^+}=2n_{H_2SO_4}=0,04\left(mol\right)\)
\(n_{NaOH}=0,3.0,4=0,12\left(mol\right)=n_{Na^+}=n_{OH^-}\)
\(\Rightarrow\sum n_{H^+}=0,02+0,04=0,06\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
0,06__0,06 (mol)
⇒ nOH- dư = 0,12 - 0,06 = 0,06 (mol)
\(\Rightarrow\left\{{}\begin{matrix}\left[Cl^-\right]=\dfrac{0,02}{0,1+0,3}=0,05\left(M\right)\\\left[SO_4^{2-}\right]=\dfrac{0,02}{0,1+0,3}=0,05\left(M\right)\\\left[Na^+\right]=\dfrac{0,12}{0,1+0,3}=0,3\left(M\right)\\\left[OH^-\right]=\dfrac{0,06}{0,1+0,3}=0,15\left(M\right)\end{matrix}\right.\)
b, pH = 14 - (-log[OH-]) ≃ 13,176

`100mL=0,1L`
`n_{H^+}=0,1.0,05.2+0,1.0,1=0,02(mol)`
`n_{SO_4^{2-}}=0,1.0,05=0,005(mol)`
`n_{OH^-}=0,1.0,2+0,1.0,1.2=0,04(mol)`
`n_{Ba^{2+}}=0,1.0,1=0,01(mol)`
`Ba^{2+}+SO_4^{2-}->BaSO_4`
Do `0,01>0,005->` Tính theo `SO_4^{2-}`
`n_{BaSO_4}=n_{SO_4^{2-}}=0,005(mol)`
`->m_↓=0,005.233=1,165(g)`
`H^{+}+OH^{-}->H_2O`
Do `0,02<0,04->OH^-` dư
`n_{OH^{-}\ pu}=n_{H^+}=0,02(mol)`
`->n_{OH^{-}\ du}=0,04-0,02=0,02(mol)`
Trong X: `[OH^-]={0,02}/{0,1+0,1}=0,1M`
`->pH=14-pOH=14+lg[OH^-]=13`
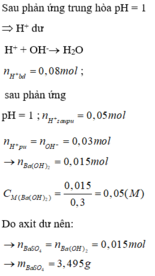
a, \(n_{HCl}=0,2.0,1=0,02\left(mol\right)=n_{H^+}=n_{Cl^-}\)
\(n_{H_2SO_4}=0,2.0,15=0,03\left(mol\right)=n_{SO_4^{2-}}\) \(\Rightarrow n_{H^+}=2n_{H_2SO_4}=0,06\left(mol\right)\)
\(\Rightarrow\Sigma n_{H^+}=0,02+0,06=0,08\left(mol\right)\)
\(n_{Ba\left(OH\right)_2}=0,3.0,05=0,015\left(mol\right)=n_{Ba^{2+}}\)
\(\Rightarrow n_{OH^-}=2n_{Ba\left(OH\right)_2}=0,03\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
0,03___0,03 (mol) ⇒ nH+ dư = 0,05 (mol)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\)
0,015___0,015______0,015 (mol) ⇒ nSO42- dư = 0,015 (mol)
⇒ m = mBaSO4 = 0,015.233 = 3,495 (g)
\(\left[Cl^-\right]=\dfrac{0,02}{0,2+0,3}=0,04\left(M\right)\)
\(\left[H^+\right]=\dfrac{0,05}{0,2+0,3}=0,1\left(M\right)\)
\(\left[SO_4^{2-}\right]=\dfrac{0,015}{0,2+0,3}=0,03\left(M\right)\)
b, pH = -log[H+] = 1