Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Cl_2}=\dfrac{13.44}{22.4}=0.6\left(mol\right)\)
\(n_{H_2}=\dfrac{11.2}{22.4}=0.5\left(mol\right)\)
\(n_{Fe}=a\left(mol\right),n_{Al}=b\left(mol\right)\)
\(n_{H_2}=a+1.5b=0.5\left(mol\right)\)
\(n_{Cl_2}=1.5a+1.5b=0.6\left(mol\right)\)
\(\Rightarrow a=b=0.2\)
\(m_X=0.2\cdot\left(56+27\right)=16.6\left(g\right)\)

Gọi số mol Al, Fe là a, b (mol)
=> 27a + 56b = 11,1 (1)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
a----------------------->1,5a
Fe + 2HCl --> FeCl2 + H2
b------------------------>b
=> 1,5a + b = 0,3 (2)
(1)(2) => a = 0,1; b = 0,15
=> \(\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1.27}{11,1}.100\%=24,32\%\\\%m_{Fe}=\dfrac{0,15.56}{11,1}.100\%=75,68\end{matrix}\right.\)

2al+6hcl-> 2alcl3+ 3h2
fe+2hcl-> fecl2+h2
nh2=13,44/22,4=0,6 mol
27a+56b=16,5
1,5a+ b=0,6
a=0,3, b=0,15
%mal=0,3*27/16,5*100=49,09%
%mfe=50,9%
nhcl=3a+2b=1,2
Vdd hcl=1,2/2=0,6l

Fe+2HCl->FeCl2+H2
x---2x-----------x
Mg+2HCl->MgCl2+H2
y------2y-----------y
Ta có :
\(\left\{{}\begin{matrix}56x+24y=24\\x+y=\dfrac{13,44}{22,4}\end{matrix}\right.\)
=>x=0,3 mol, y=0,3 mol
=>%m Fe=\(\dfrac{0,3.56}{24}.100\)=70%
=>%m Mg=100-70=30%
=>VHCl=\(\dfrac{0,3.2+0,3.2}{2}\)=0,6l=600ml
b)
XCl2+2AgNO3->2AgCl+X(NO3)2
0,6--------------------1,2mol
=>m AgCl=1,2.143,5=172,2g

Sửa: $V_{H_2}=7,168(l)$
$a\bigg)$
Đặt $n_{Mg}=x;n_{Fe}=y;n_{Al}=z$
$\to 24x+56y+27z=9,52(1)$
$n_{H_2}=\dfrac{7,168}{22,4}=0,32(mol)$
$n_{Cl_2}=\dfrac{8,064}{22,4}=0,36(mol)$
BTe: $x+y+1,5z=n_{H_2}=0,32(2)$
BTe: $x+1,5y+1,5z=n_{Cl_2}=0,36(3)$
Từ $(1)(2)(3)\to x=0,12(mol);y=0,08(mol);z=0,08(mol)$
$\to \begin{cases} \%m_{Mg}=\dfrac{0,12.24}{9,52}.100\%=30,25\%\\ \%m_{Fe}=\dfrac{0,08.56}{9,52}.100\%=47,06\%\\ \%m_{Al}=100-47,06-30,25=22,69\% \end{cases}$
$b\bigg)$
Bảo toàn H: $n_{HCl}=2n_{H_2}=0,64(mol)$
$\to C_{M_{HCl}}=\dfrac{0,64}{0,2}=3,2M$
$\to a=3,2$
$c\bigg)$
Dung dịch sau gồm $MgCl_2,FeCl_2,AlCl_3$
Bảo toàn $Mg,Al,Fe:n_{MgCl_2}=0,12(mol);n_{AlCl_3}=n_{FeCl_2}=0,08(mol)$
$\to C_{M_{MgCl_2}}=\dfrac{0,12}{0,2}=0,6M$
$\to C_{M_{AlCl_3}}=C_{M_{FeCl_2}}=\dfrac{0,08}{0,2}=0,4M$
$a\bigg)$
Đặt $n_{Mg}=x;n_{Fe}=y;n_{Al}=z$
$\to 24x+56y+27z=9,52(1)$
$n_{H_2}=\dfrac{14,336}{22,4}=0,64(mol)$
$n_{Cl_2}=\dfrac{8,064}{22,4}=0,36(mol)$
BTe: $x+y+1,5z=n_{H_2}=0,64(2)$
BTe: $x+1,5y+1,5z=n_{Cl_2}=0,36(3)$
Từ $(1)(2)(3)\to$ nghiệm âm, xem lại đề

\(n_{HCl}=0.5\cdot1=0.5\left(mol\right)\)
\(n_{H_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(0.15.....0.3........................0.15\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(0.1.........0.5-0.3\)
\(m_A=0.15\cdot24+0.1\cdot80=11.6\left(g\right)\)
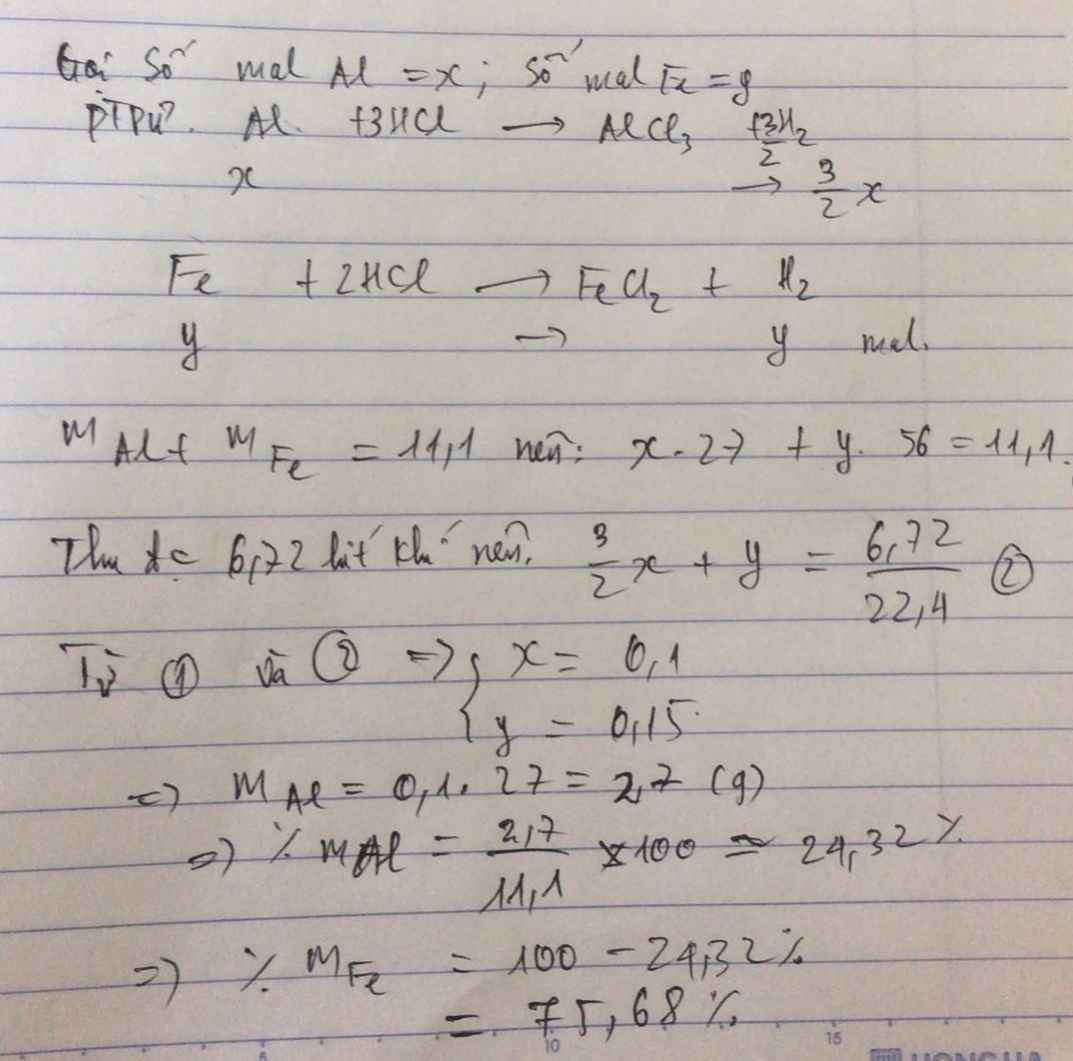

\(Cl_2+2NaBr\rightarrow2NaCl+Br_2\\Cl_2+2NaI\rightarrow2NaCl+I_2\\ n_{NaBr}=1.0,2=0,2\left(mol\right)\\ n_{NaI}=0,2.2=0,4\left(mol\right)\\ n_{Cl_2}=\dfrac{n_{NaBr}+n_{NaI}}{2}=\dfrac{0,2+0,4}{2}=0,3\left(mol\right)\\ V_{Cl_2\left(đktc\right)}=0,3.22,4=6,72\left(lít\right)\)