Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đổi: 24kg = 24000g
24kg than đá có chứa 0,5% tạp chất lưu huỳnh và 1,5% tạp chất khác không cháy được

⇒ nS = 120 / 32 = 3,75 mol
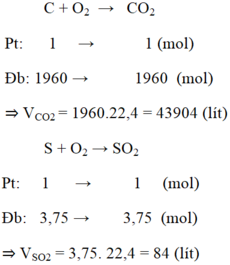

PT: \(C+O_2\underrightarrow{t^o}CO_2\) (1)
\(S+O_2\underrightarrow{t^o}SO_2\) (2)
Ta có: mS = 24.0,5% = 0,12 (kg) = 120 (g) ⇒ nS = 120/32 = 3,75 (mol)
Theo PT (2): \(n_{SO_2}=n_S=3,75\left(mol\right)\)
\(\Rightarrow V_{SO_2}=3,75.22,4=84\left(l\right)\)
Ta có: mC = 24 - 0,12 - 24.1,5% = 23,52 (kg) = 23520 (g)
\(\Rightarrow n_C=\dfrac{23520}{12}=1960\left(mol\right)\)
Theo PT (1): \(n_{CO_2}=n_C=1960\left(mol\right)\)
\(\Rightarrow V_{CO_2}=1960.22,4=43904\left(l\right)\)
Sửa: mC = 23,52 (kg) = 23420 (g)
⇒ \(n_C=\dfrac{23520}{12}=1960\left(mol\right)\)
Theo PT (1): \(n_{CO_2}=n_C=1960\left(mol\right)\)
\(\Rightarrow V_{CO_2}=1960.22,4=43904\left(l\right)\)

\(m_S=0,5\%.24=0,12\left(kg\right)=120\left(g\right)\\ \Rightarrow n_S=\dfrac{120}{32}=3,75\left(mol\right)\\ m_C=1,5\%.24=0,36\left(kg\right)=360\left(g\right)\\ \Rightarrow n_C=\dfrac{360}{12}=30\left(mol\right)\\ S+O_2\rightarrow\left(t^o\right)SO_2\\ C+O_2\rightarrow\left(t^o\right)CO_2\\ n_{SO_2}=n_S=3,75\left(mol\right)\\ \Rightarrow V_{SO_2\left(đktc\right)}=3,75.22,4=84\left(l\right)\\ n_{CO_2}=n_C=30\left(mol\right)\\ \Rightarrow V_{CO_2\left(đktc\right)}=22,4.30=672\left(l\right)\)
(Chắc đề là 1,5% C)

mS= 0,5% . 24=0,12(kg)=120(g)
->nS= 120/32=3,75(mol)
mC=(100% - 2%). 24=23,52(kg)=23520(g)
-> nC= 23520/12=1960(mol)
PTHH: S + O2 -to-> SO2
3,75______________3,75(mol)
C + O2 -to-> CO2
1960______1960(mol)
=> V(SO2,đktc)=3,75 x 22,4=84(l)
V(CO2,đktc)= 1960 x 22,4= 43904(l)

Ta có: \(m_C=1,5.1000.90\%=1350\left(g\right)\)
\(n_C=\dfrac{1350}{12}=112,5\left(mol\right)\)
PT: \(C+O_2\underrightarrow{t^o}CO_2\)
Theo PT: \(n_{O_2}=n_C=112,5\left(mol\right)\)
\(\Rightarrow V_{O_2}=112,5.22,4=2520\left(l\right)\)
\(V_{kk}=V_{O_2}.5=12600\left(l\right)\)

0,5g = 0,0005 kg
\(m_C=36-0,0005-\left(36.1,5\%\right)=35,4595kg=35459,5g\)
\(n_C=\dfrac{35459,5}{12}=2954,95mol\)
\(n_S=\dfrac{0,5}{32}=0,015625mol\)
\(C+O_2\rightarrow\left(t^o\right)CO_2\)
2954,5 2954,5 ( mol )
\(S+O_2\rightarrow\left(t^o\right)SO_2\)
\(0,015625\) \(0,015625\) ( mol )
\(V_{CO_2}=2954,5.22,4=66180,8l\)
\(V_{SO_2}=\)\(0,015625.22,4=0,35l\)

$m_S = 24.0,5\% = 0,12(kg)$
$m_C = 24.(100\% - 0,5\% - 1,5\%) = 23,52(kg)$
$\Rightarrow n_S = 0,00375(kmol) = 3,75(mol)$
$n_C = 1,96(kmol) = 1960(mol)$
$C + O_2 \xrightarrow{t^o} CO_2$
$S + O_2 \xrightarrow{t^o} SO_2$
Theo PTHH :
$n_{CO_2} = n_C = 1960(mol) ; n_{SO_2} = n_S = 3,75(mol)$
$\Rightarrow V_{CO_2} = 1960.22,4 = 43904(lít)$
$\Rightarrow V_{SO_2} = 3,75.22,4 = 84(lít)$

\(m_C=12\cdot\left(100-1.5-0.5\right)\%=11.76\left(kg\right)\)
\(n_C=\dfrac{11.76}{12}=0.98\left(kmol\right)\)
\(m_S=12\cdot0.5=6\left(kg\right)\)
\(n_S=\dfrac{6}{32}=0.1875\left(kmol\right)\)
\(S+O_2\underrightarrow{t^0}SO_2\)
\(C+O_2\underrightarrow{t^0}CO_2\)
\(V_{O_2}=\left(0.1875+0.98\right)\cdot22.4=26.152\left(kl\right)=26125\left(l\right)\)
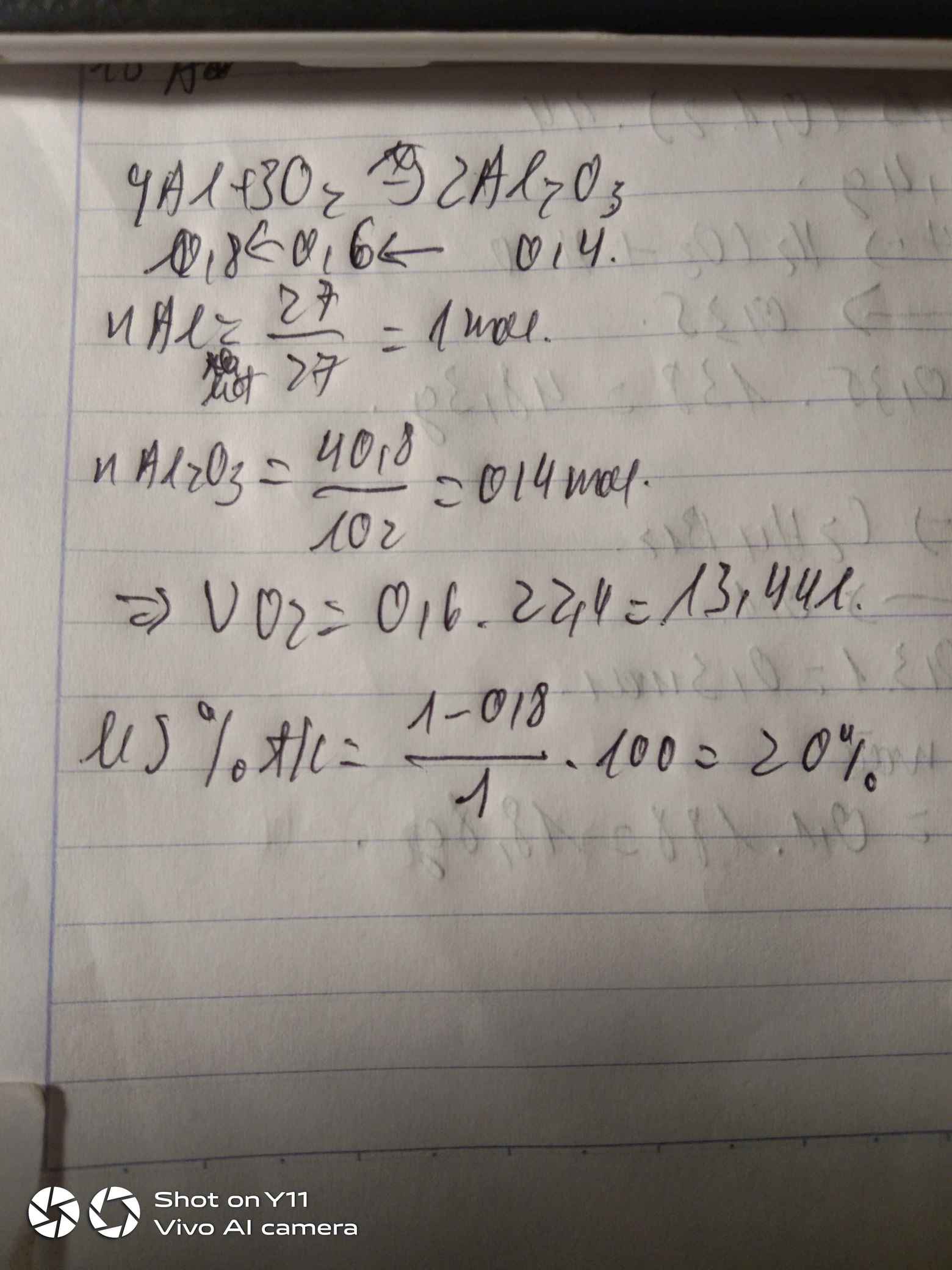

\(0,8\left(kg\right)=800\left(g\right)\)
\(m_{CaCO_3}=\dfrac{800.\left(100-10\right)}{100}=720\left(g\right)\)
\(n_{CaCO_3}=\dfrac{720}{100}=7,2\left(mol\right)\)
\(CaCO_3\rightarrow\left(t^o\right)CaO+CO_2\)
7,2 7,2 ( mol )
\(V_{CO_2}=7,2.22,4=161,28\left(l\right)\)