Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Câu 2:
Trong 1 mol X: \(\left\{{}\begin{matrix}n_{Ag}=\dfrac{170.63,53\%}{108}=1\left(mol\right)\\n_N=\dfrac{170.8,23\%}{14}=1\left(mol\right)\\n_O=\dfrac{170\left(100\%-63,53\%-8,23\%\right)}{16}=3\left(mol\right)\end{matrix}\right.\)
Vậy CTHH của X là \(AgNO_3\)
Câu 1:
\(a,\%_{Fe}=\dfrac{56}{180}\cdot100\%=31,11\%\\ \%_N=\dfrac{14\cdot2}{180}\cdot10\%=15,56\%\\ \%_O=100\%-31,11\%-15,56\%=53,33\%\\ b,\%_{N\left(N_2O\right)}=\dfrac{14\cdot2}{44}\cdot100\%=63,63\%\\ \%_{O\left(N_2O\right)}=100\%-63,63\%=36,37\%\\ \%_{N\left(NO\right)}=\dfrac{14}{30}\cdot100\%=46,67\%\\ \%_{O\left(NO\right)}=100\%-46,67\%=53,33\%\\ \%_{O\left(NO_2\right)}=\dfrac{16\cdot2}{46}\cdot100\%=69,57\%\\ \%_{N\left(NO_2\right)}=100\%-69,57\%=30,43\%\)

\(\%m_{Ca}=\dfrac{40}{164}.100=24,39\left(\%\right)\\ \%m_N=\dfrac{28}{164}.100=17,07\left(\%\right)\\ \%m_O=\dfrac{48.2}{164}.100=58,54\left(\%\right)\)

Đ
ặ
t
:
Y
(
N
O
3
)
2
V
ì
:
%
m
Y
=
34
,
043
%
⇔
M
Y
M
Y
+
124
=
34
,
043
%
⇔
M
Y
=
64
(
g
m
o
l
)
⇒
Y
:
Đ
ồ
n
g
(
C
u
=
64
)
⇒
C
T
H
H
:
C
u
(
N
O
3
)
2
Thu gọn

\(\%N\left(CO\left(NH_2\right)_2\right)=\dfrac{2.14}{60}.100\%=46,67\%\)
\(\%N\left(\left(NH_4\right)_2SO_4\right)=\dfrac{2.14}{132}.100\%=21,21\%\)
\(\%N\left(NH_4NO_3\right)=\dfrac{2.14}{80}.100\%=35\%\)
\(\%N\left(Ca\left(NO_3\right)_2\right)=\dfrac{2.14}{164}.100\%=17,07\%\)
=> CO(NH2)2 có hàm lượng N cao nhất
=> A

\(Fe\left(NO_3\right)_3:\left\{{}\begin{matrix}\%_{Fe}=\dfrac{56}{242}\cdot100\%=23,14\%\%\\\%_N=\dfrac{14\cdot3}{242}\cdot100\%=17,36\%\\\%_O=\left(100-23,14-17,36\right)\%=59,5\%\end{matrix}\right.\)
\(K_3PO_4:\left\{{}\begin{matrix}\%_K=\dfrac{39\cdot3}{212}\cdot100\%=55,19\%\\\%_P=\dfrac{31}{212}\cdot100\%=14,62\%\\\%_O=\left(100-55,19-14,62\right)\%=30,19\%\end{matrix}\right.\)
\(Ca\left(OH\right)_2:\left\{{}\begin{matrix}\%_{Ca}=\dfrac{40}{74}\cdot100\%=54,05\%\\\%_O=\dfrac{16\cdot2}{74}\cdot100\%=43,24\%\\\%_H=\left(100-54,05-43,24\right)\%=2,71\%\end{matrix}\right.\)
\(P_2O_5:\left\{{}\begin{matrix}\%_P=\dfrac{31\cdot2}{142}\cdot100\%=43,66\%\\\%_O=100\%-43,66\%=56,34\%\end{matrix}\right.\\ SiO_2:\left\{{}\begin{matrix}\%_{Si}=\dfrac{28}{60}\cdot100\%=46,67\%\\\%_O=\left(100-46,67\right)\%=53,33\%\end{matrix}\right.\\ Fe_3O_4:\left\{{}\begin{matrix}\%_{Fe}=\dfrac{56\cdot3}{232}\cdot100\%=72,41\%\\\%_O=\left(100-72,41\right)\%=27,59\%\end{matrix}\right.\)

\(M_{MgSO_4}=24+32+16.4=120\\ \%Mg=\dfrac{24}{120}.100=20\%\\ \%S=\dfrac{32}{120}.100=26,67\%\\ \%O=\dfrac{16.4}{120}.100=53,33\%\\ M_{Al\left(NO_3\right)_3}=27+62.3=213\\ \%Al=\dfrac{27}{213}.100=12,68\%\\ \%N=\dfrac{14.3}{213}.100=19,72\%\\ \%O=\dfrac{16.9}{213}.100=67,6\%\)

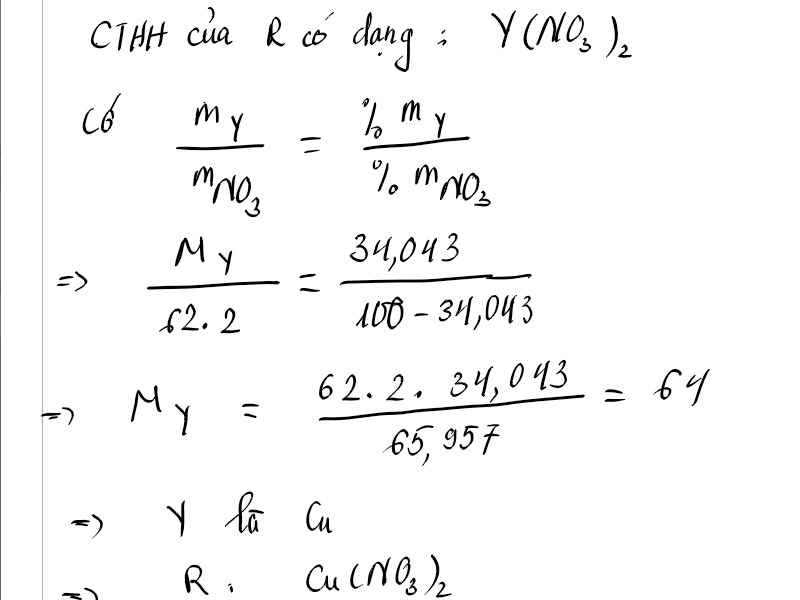
a) ta có : MCO(NH2)2 = 64(g/mol)
=> %mC=12/64.100=18,75%
%mO=16/64.100=25%
%mN=14.2/64.100=43,75%
%mH=2.2.2/64.100=12,5%
b) MCa(NO3)2=164(g/mol)
=>%mCa=40/164.100=24,39%
%mN=14.2/164.100=17,07%
%mO=16.3.2/164.100=58,53%