Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

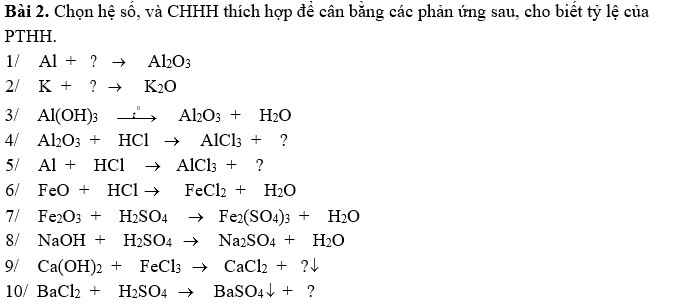
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\\ 4K+O_2\underrightarrow{t^o}2K_2O\\ 2Al\left(OH\right)_3\underrightarrow{t^o}Al_2O_3+3H_2O\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
\(FeO+2HCl\rightarrow FeCl_2+2H_2O\)
\(Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
\(3Ca\left(OH\right)_2+2FeCl_3\rightarrow3CaCl_2+2Fe\left(OH\right)_3\)
\(BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\)

\(=40+12+\left(16.3\right)=52+48=100\left(dvC\right)\)

\(n_{K_2SO_3}=\dfrac{15.8}{158}=0.1\left(mol\right)\)
\(K_2SO_3+H_2SO_4\rightarrow K_2SO_4+SO_2+H_2O\)
\(0.1...........................0.1..........0.1\)
\(V_{SO_2}=0.1\cdot22.4=2.24\left(l\right)\)
\(C_{M_{K_2SO_4}}=\dfrac{0.1}{0.2}=0.5\left(M\right)\)

a, \(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PTHH: Fe + H2SO4 → FeSO4 + H2
Mol: 0,25 0,25 0,25 0,25
\(m_{Fe}=0,25.56=14\left(g\right)\)
\(m_{H_2SO_4}=0,25.98=24,5\left(g\right)\)
b, \(m_{FeSO_4}=0,25.162=40,5\left(g\right)\)


\(n_{O_2}=\dfrac{6,4}{32}=0,2\left(mol\right)\)
\(n_{CO_2}=\dfrac{13,2}{44}=0,3\left(mol\right)\)
=> \(\overline{M}=\dfrac{6,4+13,2}{0,2+0,3}=39,2\left(g/mol\right)\)

\(a,n_{CaO}=\dfrac{14}{40}=0,35(mol)\\ b,n_{C}=\dfrac{3.10^{-23}}{6.10^{-23}}=0,5(mol)\\ c,n_{H_2O}=\dfrac{9.10^{-23}}{6.10^{-23}}=1,5(mol)\\ d,n_{O_2}=\dfrac{16}{32}=0,5(mol)\)

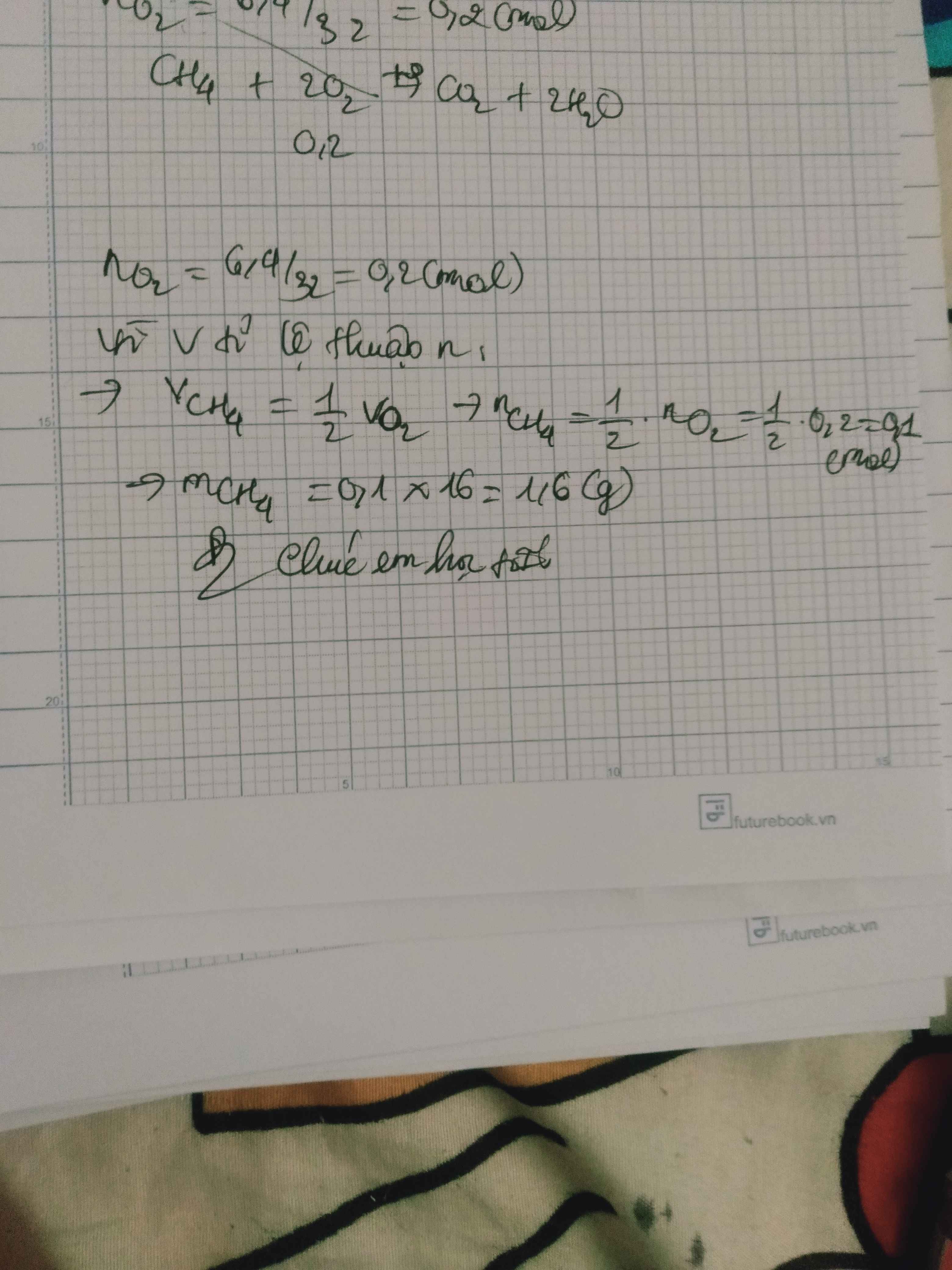
\(n_{O_2}=\dfrac{6,4}{32}=0,2mol\)