
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


24.
10
23
phân tử
H
2
O
=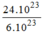 = 4(mol) phân tử
H
2
O
= 4(mol) phân tử
H
2
O
1,44.
10
23
phân tử
C
O
2
=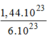 = 0,24(mol) phân tử
C
O
2
.
= 0,24(mol) phân tử
C
O
2
.
0,66.
10
23
phân tử
C
12
H
22
O
11
=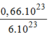 = 0,11(mol) phân tử
C
12
H
22
O
11
.
= 0,11(mol) phân tử
C
12
H
22
O
11
.

Số mol là: \(\dfrac{3.10^{23}}{6.10^{23}}=\dfrac{3}{6}=\dfrac{1}{2}=0,5\left(mol\right)\)

\(n_{H_2O}=\dfrac{24.10^{23}}{6.10^{23}}=4\left(mol\right)\\ n_{CO_2}=\dfrac{1,44.10^{23}}{6.10^{23}}=0,24\left(mol\right)\\ n_{Fe}=\dfrac{1,2.10^{23}}{6.10^{23}}=0,2\left(mol\right)\\ n_C=\dfrac{0,66.10^{23}}{6.10^{23}}=0,11\left(mol\right)\)

\(1.m_{Cu}=1,2.64=76,8\left(g\right)\\ 2.m_{NaCl}=1,25.58,5=73,125\\ 3.n_{C_6H_{12}O_6}=\dfrac{7,2.10^{23}}{6.10^{23}}=1,2\left(mol\right)\\ \Rightarrow m_{C_6H_{12}O_6}=1,2.180=216\left(g\right)\\ 4.n_{O_2}=3,6.32=115,2\left(g\right)\\ 5.n_{O_2}=\dfrac{1,2.10^{23}}{6.10^{23}}=0,2\left(mol\right)\\ \Rightarrow m_{O_2}=0,2.32=6,4\left(g\right)\\ 6.n_{N_2}=\dfrac{26,88}{22,4}=1,2\left(mol\right)\\ \Rightarrow m_{N_2}=1,2.28=33,6\left(g\right)\\ 7.n_{CO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\\ \Rightarrow m_{CO_2}=0,5.44=22\left(g\right)\\ 8.n_{H_2}=\dfrac{31,36}{22,4}=1,4\left(mol\right)\\ \Rightarrow m_{H_2}=1,4.2=2,8\left(g\right)\)
\(1,m_{Cu}=1,2\cdot64=76,8\left(g\right)\\ 2,m_{NaCl}=1,25\cdot58,5=73,125\left(g\right)\\ 3,n_{C_6H_{12}O_6}=\dfrac{7,2\cdot10^{-23}}{6\cdot10^{-23}}=1,2\left(mol\right)\\ \Rightarrow m_{C_6H_{12}O_6}=1,2\cdot180=216\left(g\right)\\ 4,m_{O_2}=3,6\cdot32=115,2\left(g\right)\\ 5,n_{O_2}=\dfrac{1,2\cdot10^{-23}}{6\cdot10^{-23}}=0,2\left(mol\right)\\ \Rightarrow m_{O_2}=0,2\cdot32=6,4\left(g\right)\\ 6,n_{N_2}=\dfrac{26,88}{22,4}=1,2\left(mol\right)\\ \Rightarrow m_{N_2}=1,2\cdot28=33,6\left(g\right)\\ 7,n_{CO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\\ \Rightarrow m_{CO_2}=0,5\cdot44=22\left(g\right)\\ 8,n_{H_2}=\dfrac{31,36}{22,4}=1,4\left(mol\right)\\ \Rightarrow m_{H_2}=1,4\cdot2=2,8\left(g\right)\)
Số mol của Fe: \(n_{Fe}=\frac{4,5.10^{23}}{6.10^{23}}=0,75\left(mol\right)\)
nFe = 4,5.1023/6.1023 = 0,75 mol