Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1
\(a,n_{CuO}=\dfrac{16}{80}=0,2\left(mol\right)\\ CuO+2HCl\xrightarrow[]{}CuCl_2+H_2O\\ n_{CuCl_2}=n_{CuO}=0,2mol\\ m_{CuCl_2}=0,2.135=27\left(g\right)\\ b.n_{HCl}=0,2.2=0,4\left(mol\right)\\ C_{MHCl}=\dfrac{0,4}{0,5}=0,8\left(M\right)\)
Bài 5
\(a,n_{NaOH}=0,2.1=0,2\left(mol\right)\\ 2NaOH+H_2SO_4\xrightarrow[]{}Na_2SO_4+2H_2O\\ n_{H_2SO_4}=0,2:2=0,1\left(mol\right)\\ C_{MH_2SO_4}=\dfrac{0,1}{0,4}=0,25\left(M\right)\\ b,n_{Na_2SO_4}=0,2:2=0,1\left(mol\right)\\ C_{MNa_2SO_4}=\dfrac{0,1}{0,2+0,4}=\dfrac{1}{6}\left(M\right)\\ c,m_{Na_2SO_4}=0,1.142=14,2\left(g\right)\)

\(a/\\MgO+2HCl \to MgCl_2+H_2O\\ n_{MgO}=\frac{8}{40}=0,2(mol)\\ b/\\ n_{HCl}=0,2.2=0,4(mol)\\ CM_{HCl}=\frac{0,4}{0,2}=2M\)

\(nCuO=\dfrac{80}{80}=1\left(mol\right)\)
\(2CH_3COOH+CuO\rightarrow\left(CH_3COO\right)_2Cu+H_2O\)
2 1 1 1 (mol)
\(mCH_3COOH=2.60=120\left(g\right)\)
m muối = \(m\left(CH_3COO\right)_2Cu=1.182=182\left(g\right)\)
m H2O = 1.18 = 18 (g)
mdd = mddCH3COOH + m(CH3COO)2Cu + mH2O - mCuO
= 100 + 182 + 18 - 80 = 220 (g)
\(C\%_{ddCH_3COOH}=\dfrac{120.100}{220}=54,55\%\)
a) \(n_{CuO}=\dfrac{80}{80}=1\left(mol\right)\)
PTHH: CuO + 2CH3COOH ---> (CH3COO)2Cu + H2O
1---->2--------------------->1
=> mmuối = 1.182 = 182 (g)
b) \(C\%_{CH_3COOH}=\dfrac{60.2}{100}.100\%=120\%\) đề có sai không vậy bạn ?

PTHH: \(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(CuCl_2+2KOH\rightarrow2KCl+Cu\left(OH\right)_2\downarrow\)
a+b) Ta có: \(n_{CuO}=\dfrac{8}{80}=0,1\left(mol\right)\)
\(\Rightarrow n_{HCl}=0,2\left(mol\right)=n_{KOH}\) \(\Rightarrow\left\{{}\begin{matrix}C\%_{HCl}=\dfrac{0,2\cdot36,5}{300}\cdot100\%\approx2,43\%\\C_{M_{KOH}}=\dfrac{0,2}{0,2}=1\left(M\right)\end{matrix}\right.\)
c) PTHH: \(Cu\left(OH\right)_2\xrightarrow[]{t^o}CuO+H_2O\)
Theo các PTHH: \(n_{CuO\left(lý.thuyết\right)}=n_{Cu\left(OH\right)_2}=n_{Cu}=0,1\left(mol\right)\)
\(\Rightarrow n_{CuO}=0,1\cdot95\%=0,095\left(mol\right)\) \(\Rightarrow m_{CuO}=0,095\cdot80=7,6\left(g\right)\)

nFe2O3=0.1(mol)
PTHH Fe2O3+6HCl->2FeCl3+3H2O
a)Theo pthh,nHCl=6 nFe2O3->nHCl =0.1*6=0.6(mol)
mHCl=0.6*36.5=21.9(g)
b)nFeCl3=0.2(mol)
mFeCl3=162.5*0.2=32.5(g)
mdd sau phản ứng:248+16=264(g)
C%muối=32.5:264*100=12.3%
nFe2O3 = 16/160 = 0,1 mol
a/ Fe2O3 + 6HCl -----> 2FeCl3 + 3H2O
(mol) 0,1 0,6 0,2
b/ Từ PTHH => nHCl = 6nFe2O3 = 0,6 mol
=> mHCl = 0,6 x 36,5 = 21,9 (g)
c/ nFeCl3 = 2nFe2O3 = 0,2 mol
=> mFeCl3 = 0,2 x 162,5 = 32,5 (g)
=> %FeCl3 = \(\frac{32,5}{248}.100\approx13,105\%\)

a, PTHH: \(Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O\)
b. Ta có \(n_{Fe_2O_3}=\frac{16}{160}=0,1\) (mol)
Theo PTHH: \(n_{HCl}=6n_{Fe_2O_3}=6.0,1=0,6\) (mol)
=> \(m_{HCl}=0,6.36,5=21,9\) (g)
c, Theo PTHH: n FeCl3 = 0,2 (mol)
=> m FeCl3 = 0,2 . 162,5 =32,5 (g)
Áp dụng ĐLBTKL ta có:
\(m_{dd-sau-p.ư}=m_{Fe_2O_3}+m_{ddHCl}=16+248=264\left(g\right)\)
=> C% FeCl3 = \(\frac{32,5}{264}.100\%\approx12,31\%\)

a, \(Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O\)
\(FeCl_3+3KOH\rightarrow3KCl+Fe\left(OH\right)_{3\downarrow}\)
\(n_{Fe_2O_3}=\dfrac{16}{160}=0,1\left(mol\right)\)
Theo PT: \(n_{HCl}=6n_{Fe_2O_3}=0,6\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,6.36,5=21,9\left(g\right)\)
b, \(n_{Fe\left(OH\right)_3}=n_{FeCl_3}=2n_{Fe_2O_3}=0,2\left(mol\right)\)
\(\Rightarrow m_{Fe\left(OH\right)_3}=0,2.107=21,4\left(g\right)\)
\(n_{KOH}=3n_{FeCl_3}=0,6\left(mol\right)\)
\(\Rightarrow C_{M_{KOH}}=\dfrac{0,6}{0,2}=3\left(M\right)\)
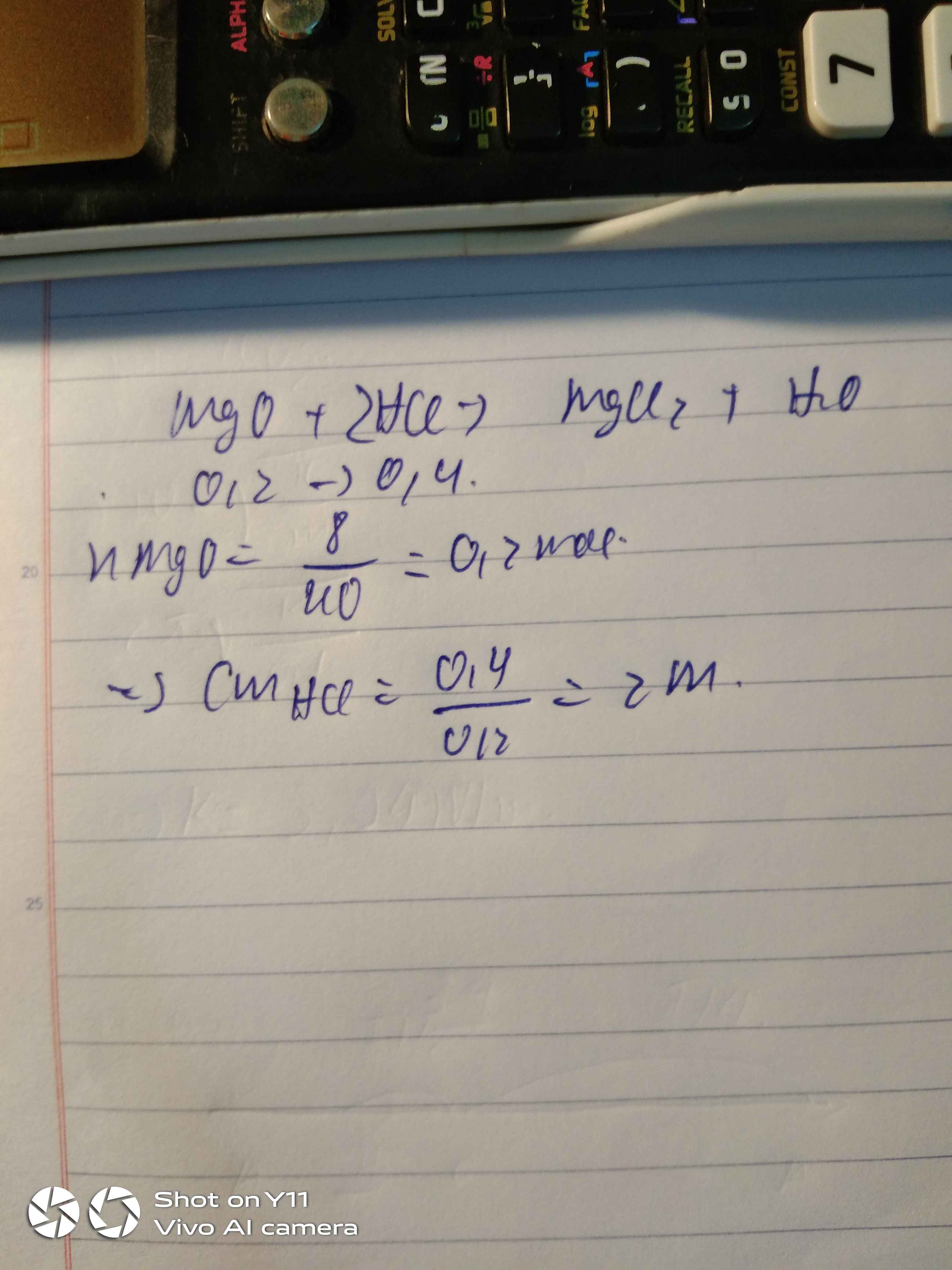
a. PTHH: CuO + 2HCl ---> CuCl2 + H2O
0,25 0,5 0,25 (mol)
Ta có: n CuO = 16/64 = 0,25 ( mol)
Theo pthh: n CuCl2 = 0,25 (mol)
=> m CuCl2 = 0,25 ( 64 + 35,5.2 ) = 33,75 (g)
b, Theo pthh: n HCl = 0,5 (mol)
=> \(C_{M_{HCl}}=\frac{0,5}{0,1}=5M\)
nCuO=0.2(mol)
CuO+2HCl->CuCl2+H2O
nCuCl2=nCuO->nCuCl2=0.2(mol)
mCuCl2=27(g)
nHCl=2 nCuO->nHCl=0.4(mol)
CM=0.4:0.1=4(M)