Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ Đặt:n_{Fe}=a\left(mol\right);n_{Zn}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}56a+65b=23,3\\a+b=0,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,3\\b=0,1\end{matrix}\right.\\ \Rightarrow\%m_{Zn}=\dfrac{0,1.65}{23,3}.100\approx27,897\%\\ \Rightarrow\%m_{Fe}\approx72,103\%\)
Fe+2HCl--->FeCl2+H2
Zn+2HCl-->ZnCl2+H2
Gọi số mol của Fe và Zn lần lượt là x,y mol
=> ta có hpt {56x+65y=23,3
{x+y=8,96/22,4
<=>{x=0,3=>mFe=16,8g
{y=0,1=>mZn=6,5g
nHCl=2nH2=2.8,96/22,4=0,8 mol
=>mHCl=29,2g
%mFe=16,8/23,3.100=72,10300429%
=>%mZn=27,89699571%
Chúc bn học giỏi

a) \(n_{AlCl_3}=\dfrac{6,675}{133,5}=0,05\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
0,05<-----------0,05---->0,075
=> \(\%Al=\dfrac{0,05.27}{14,15}.100\%=9,54\%\)
=> \(\%Cu=\dfrac{14,15-0,05.27}{14,15}.100\%=90,46\%\)
b) \(V_{H_2}=0,075.22,4=1,68\left(l\right)\)
c) \(n_{Cu}=\dfrac{14,15-0,05.27}{64}=0,2\left(mol\right)\)
PTHH: 4Al + 3O2 --to--> 2Al2O3
0,05->0,0375
2Cu + O2 --to--> 2CuO
0,2-->0,1
=> \(V_{O_2}=\left(0,1+0,0375\right).22,4=3,08\left(l\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\\ m_{AlCl_3}=6,675\left(mol\right)\\ n_{AlCl_3}=\dfrac{6,675}{133,5}=0,05\left(mol\right)\\ \Rightarrow n_{Al}=n_{AlCl_3}=0,05\left(mol\right)\\ \Rightarrow m_A=0,05.27=1,35\left(g\right);m_{Cu}=14,15-1,35=12,8\left(g\right)\\ \%m_{Cu}=\dfrac{12,8}{14,15}.100\approx90,459\%\\ \Rightarrow\%m_{Al}\approx9,541\%\\ b,n_{Cu}=\dfrac{12,8}{64}=0,2\left(mol\right)\\ n_{H_2}=\dfrac{3}{2}.n_{Al}=\dfrac{3}{2}.0,05=0,075\left(mol\right)\\ \Rightarrow V=V_{H_2\left(đktc\right)}=0,075.22,4=1,68\left(l\right)\\ 4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ 2Cu+O_2\rightarrow\left(t^o\right)2CuO\\ n_{O_2}=\dfrac{3}{4}.n_{Al}+\dfrac{1}{2}.n_{Cu}=\dfrac{3}{4}.0,05+\dfrac{1}{2}.0,2=0,0875\left(mol\right)\)
\(\Rightarrow V_{O_2\left(đktc\right)}=0,0875.22,4=1,96\left(l\right)\)

\(Fe+2HCl\rightarrow FeCl_2+H_2\\ n_{Fe}=n_{H_2}=\dfrac{0,448}{22,4}=0,02\left(mol\right)\\ a,\%m_{Fe}=\dfrac{0,02.56}{4,36}.100\approx25,688\%\\ \Rightarrow\%m_{Ag}\approx74,312\%\\ b,Ta.thấy:2,18=\dfrac{1}{2}.4,36\\ \Rightarrow m_{hh\left(câuB\right)}=\dfrac{1}{2}.m_{hh\left(câuA\right)}\\ n_{Fe}=\dfrac{0,02}{2}=0,01\left(mol\right)\\ n_{Ag}=\dfrac{2,18-0,01.56}{108}=0,015\left(mol\right)\\ 2Fe+3Cl_2\rightarrow\left(t^o\right)2FeCl_3\\ 2Ag+Cl_2\rightarrow\left(t^o\right)2AgCl\\ n_{Cl_2}=\dfrac{3}{2}.n_{Fe}+\dfrac{1}{2}.n_{Ag}=\dfrac{3}{2}.0,01+\dfrac{1}{2}.0,015=0,0225\left(mol\right)\\ \Rightarrow V_{Cl_2\left(đktc\right)}=0,0225.22,4=0,504\left(l\right)\)

a) Đặt \(\left\{{}\begin{matrix}n_{Al}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\end{matrix}\right.\) \(\Rightarrow27a+56b=11\) (1)
Ta có: \(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
Bảo toàn electron: \(3a+2b=0,4\cdot2=0,8\) (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,2\cdot27}{11}\cdot100\%\approx49,09\%\\\%m_{Fe}=50,91\%\end{matrix}\right.\)
b) Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{AlCl_3}=n_{Al}=0,2\left(mol\right)\\n_{FeCl_2}=n_{Fe}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{muối}=m_{AlCl_3}+m_{FeCl_2}=0,2\cdot133,5+0,1\cdot127=39,4\left(g\right)\)
c) Bảo toàn electron: \(3\cdot0,2+3\cdot0,1=2n_{SO_2}\)
\(\Rightarrow n_{SO_2}=0,45\left(mol\right)\) \(\Rightarrow V_{SO_2}=0,45\cdot22,4=10,08\left(l\right)\)
a) Gọi nAl = x, nFe = y
Có 27x + 56y = 11 (1)
Bảo toàn e
3x + 2y = 2.0,4 (2)
Từ 1 và 2 => x = 0,2, y = 0,1
\(\%mAl=\dfrac{0,2.27}{11}.100\%=49,09\%\)
\(\%mFe=100-49,09=50,91\%\)
b) BTKL:
m muối = mkim loại + mHCl - mH2
= 11 + 0,4.2.36,5 - 0,4.2 = 39,4g
c)
Bảo toàn e
Al => Al+3 + 3e S+6 + 2e => S+4
0,2 0,6 2x x
Fe => Fe+3 + 3e
0,1 0,3
=> 2x = 0,6 + 0,3 => x = 0,45 mol
=> VSO2 = 0,45.22,4 = 10,08 lít

a) Gọi số mol Zn, Fe là a, b (mol)
=> 65a + 56b = 8,56 (1)
\(n_{H_2}=\dfrac{3,136}{22,4}=0,14\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
a--->2a-------->a----->a
Fe + 2HCl --> FeCl2 + H2
b----->2b------->b------>b
=> a + b = 0,14 (2)
(1)(2) => a = 0,08; b = 0,06
=> \(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{0,08.65}{8,56}.100\%=60,748\%\\\%m_{Fe}=\dfrac{0,06.56}{8,56}.100\%=39,252\%\end{matrix}\right.\)
b)
nKOH = 0,2.0,1 = 0,02 (mol)
PTHH: KOH + HCl --> KCl + H2O
0,02-->0,02
=> nHCl = 0,02 + 2a + 2b = 0,3 (mol)
=> \(C_{M\left(HCl\right)}=xM=\dfrac{0,3}{0,15}=2M\)
c) m = 0,08.136 + 0,06.127 = 18,5(g)





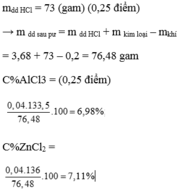
\(Đặt:\left\{{}\begin{matrix}Fe:x\left(mol\right)\\Zn:y\left(mol\right)\end{matrix}\right.\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ Tacó:\left\{{}\begin{matrix}56x+65y=5,3\\x+y=0,25\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=1,2\\y=-0,97\end{matrix}\right.\left(vô\:lí\right)\)
Em xem lại đề nha!